Consensus molecular subtyping of metastatic colorectal cancer expands biomarker-directed therapeutic benefit for patients with CMS1 and CMS2 tumors
- PMID: 39227409
- PMCID: PMC11473766
- DOI: 10.1038/s41416-024-02826-0
Consensus molecular subtyping of metastatic colorectal cancer expands biomarker-directed therapeutic benefit for patients with CMS1 and CMS2 tumors
Abstract
Background: We developed a whole transcriptome sequencing (WTS)-based Consensus Molecular Subtypes (CMS) classifier using FFPE tissue and investigated its prognostic and predictive utility in a large clinico-genomic database of CRC patients (n = 24,939).
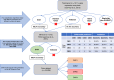
Methods: The classifier was trained against the original CMS datasets using an SVM model and validated in an independent blinded TCGA dataset (88.0% accuracy). Kaplan-Meier estimates of overall survival (OS) and time-on-treatment (TOT) were calculated for each CMS (p < 0.05 considered significant).
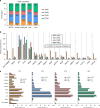
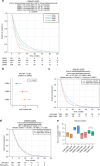
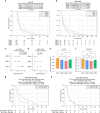
Results: CMS2 tumors were enriched on left-side of colon and conferred the longest median OS. In RAS-wildtype mCRC, left-sided tumors and CMS2 classification were associated with longer TOT with anti-EGFR antibodies (cetuximab and panitumumab). When restricting to only CMS2, there was no significant difference in TOT between right- versus left-sided tumors. CMS1 tumors were associated with a longer median TOT with pembrolizumab relative to other CMS groups, even when analyzing only microsatellite stable (MSS) tumors.
Discussion: A WTS-based CMS classifier allowed investigation of a large multi-institutional clinico-genomic mCRC cohort, suggesting anti-EGFR therapy benefit for right-sided RAS-WT CMS2 tumors and immune checkpoint inhibitor benefit for MSS CMS1. Routine CMS classification of CRC provides important treatment associations that should be further investigated.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
HL: BMS, Merck, Bayer, Oncocyte, Fulgent, 3T Bioscience, Invitae, Abalos, AffiniT, Adagene, Replimune. JM: RESEARCH SUPPORT: 2cureX, OnDose, Arcus Biosciences; PAYMENT/HONORARIA: AstraZeneca, Merck, Bayer, Seagen, Pfizer, Takeda, Taiho Pharmaceutical; CONSULTING OR ADVISORY ROLE: Caris Life Sciences; OTHER: Indivumed (CMO). GWS: MEETING SUPPORT: Caris Life Sciences; STOCK/STOCK OPTIONS: Syndax, Caris Life Sciences, Tessa Pharm. SK: RESEARCH SUPPORT: Sanofi, Biocartis, Guardant Health, Array BioPharma, Genentech/Roche, EMD Serono, MedImmune, Novartis, Amgen, Lilly, Daiichi Sankyol CONSULTNG OR ADVISORY ROLE: Genentech, EMD Serono, Merck, Holy Stone Healthcare, Novartis, Lilly, Boehringer Ingelheim, AstraZeneca/MedImmune, Bayer Health, Redx Pharma, Ipsen, HalioDx, Lutris, Jacobio, Pfizer, Repare Therapeutics, Inivata, GlaxoSmithKline, Jazz Pharmaceuticals, Iylon, Xilis, Abbvie, Amal Therapeutics, Gilead Sciences, Mirati Therapeutics, Flame Biosciences, Servier, Carina Biotech, Bicara Therapeutics, Endeavor BioMedicines, Numab, Johnson & Johnson/Janssen, Genomic Health, Frontier Medicines, Replimune, Taiho Pharmaceutical, Cardiff Oncology, Ono Pharmaceutical, Bristol-Myers Squibb-Medarex, Amgen, Tempus, Foundation Medicine, Harbinger Oncology, Inc, Takeda, CureTeq, Zentalis, Black Stone Therapeutics, NeoGenomics Laboratories, Accademia Nazionale Di Medicina, Tachyon Therapeutics; STOCK/STOCK OPTIONS: Frontier Medicines; Iylon; Lutris; Navire; Xilis. JPS: RESEARCH SUPPORT: BostonGene, Celsius Therapeutics; CONSULTING OR ADVISORY ROLE: Engine Biosciences, NaDeNo Nanoscience. JX, JRR, TN, JZ, KAP, MJO, GWS, and DS: employees of Caris Life Sciences.
Figures

References
-
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73:233–54. - PubMed
-
- Atreya CE, Yaeger R, Chu E. Systemic Therapy for Metastatic Colorectal Cancer: From Current Standards to Future Molecular Targeted Approaches. Am Soc Clin Oncol Educ Book. 2017;37:246–56. - PubMed
MeSH terms
Substances
Grants and funding
- P30 CA016672/CA/NCI NIH HHS/United States
- CA016672/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- CA234406/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- K22 CA234406/CA/NCI NIH HHS/United States
- RR180035/Cancer Prevention and Research Institute of Texas (Cancer Prevention Research Institute of Texas)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

