Targeting POLRMT by IMT1 inhibits colorectal cancer cell growth
- PMID: 39227564
- PMCID: PMC11372113
- DOI: 10.1038/s41419-024-07023-8
Targeting POLRMT by IMT1 inhibits colorectal cancer cell growth
Abstract
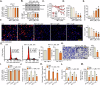
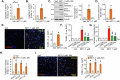
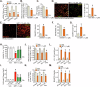
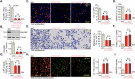
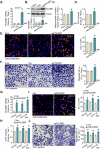
This study investigates the potential anti-colorectal cancer (CRC) activity of IMT1, a novel specific inhibitor of mitochondrial RNA polymerase (POLRMT). Single-cell RNA sequencing data reveal that POLRMT is overexpressed in CRC cells. Additionally, elevated POLRMT expression was observed in local CRC tissues and cells, while its expression remained relatively low in colon epithelial tissues and cells. IMT1 significantly inhibited colony formation, cell viability, proliferation, cell cycle progression, and migration in both primary and immortalized CRC cells. Furthermore, IMT1 induced apoptosis and cell death in CRC cells. The inhibition of POLRMT by IMT1 disrupted mitochondrial functions in CRC cells, leading to mitochondrial depolarization, oxidative damage, and decreased ATP levels. Using targeted shRNA to silence POLRMT closely mirrored the effects of IMT1, showing robust anti-CRC cell activity. Crucially, the efficacy of IMT1 was diminished in CRC cells with silenced POLRMT. Contrarily, boosting POLRMT expression externally by a lentiviral construct promoted the proliferation and migration of CRC cells. Importantly, treatment with IMT1 or silencing POLRMT in primary colon cancer cells decreased the phosphorylation of Akt1-S6K1, whereas overexpression of POLRMT had the opposite effect. In nude mice, orally administering IMT1 potently restrained primary colon cancer xenograft growth. IMT1 suppressed POLRMT activity, disrupted mitochondrial function, hindered Akt-mTOR activation, and prompted apoptosis within the xenograft tissues. In addition, IMT1 administration suppressed lung metastasis of primary colon cancer cells in nude mice. These combined results highlight the robust anti-CRC activity of IMT1 by specifically targeting POLRMT.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

