Discovery of an unconventional lamprey lymphocyte lineage highlights divergent features in vertebrate adaptive immune system evolution
- PMID: 39227584
- PMCID: PMC11372201
- DOI: 10.1038/s41467-024-51763-2
Discovery of an unconventional lamprey lymphocyte lineage highlights divergent features in vertebrate adaptive immune system evolution
Abstract
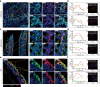
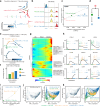
Lymphocyte receptors independently evolved in both jawed and jawless vertebrates with similar adaptive immune responses. However, the diversity of functional subtypes and molecular architecture in jawless vertebrate lymphocytes, comparable to jawed species, is not well defined. Here, we profile the gills, intestines, and blood of the lamprey, Lampetra morii, with single-cell RNA sequencing, using a full-length transcriptome as a reference. Our findings reveal higher tissue-specific heterogeneity among T-like cells in contrast to B-like cells. Notably, we identify a unique T-like cell subtype expressing a homolog of the nonlymphoid hematopoietic growth factor receptor, MPL-like (MPL-L). These MPL-L+ T-like cells exhibit features distinct from T cells of jawed vertebrates, particularly in their elevated expression of hematopoietic genes. We further discovered that MPL-L+ VLRA+ T-like cells are widely present in the typhlosole, gill, liver, kidney, and skin of lamprey and they proliferate in response to both a T cell mitogen and recombinant human thrombopoietin. These findings provide new insights into the adaptive immune response in jawless vertebrates, shedding new light on the evolution of adaptive immunity.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Bernstein, R. M., Schluter, S. F., Bernstein, H. & Marchalonis, J. J. Primordial emergence of the recombination activating gene 1 (RAG1): sequence of the complete shark gene indicates homology to microbial integrases. Proc. Natl Acad. Sci. USA93, 9454–9459 (1996). 10.1073/pnas.93.18.9454 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources

