Optoelectronic synapses with chemical-electric behaviors in gallium nitride semiconductors for biorealistic neuromorphic functionality
- PMID: 39227588
- PMCID: PMC11371922
- DOI: 10.1038/s41467-024-51194-z
Optoelectronic synapses with chemical-electric behaviors in gallium nitride semiconductors for biorealistic neuromorphic functionality
Abstract
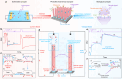
Optoelectronic synapses, leveraging the integration of classic photo-electric effect with synaptic plasticity, are emerging as building blocks for artificial vision and photonic neuromorphic computing. However, the fundamental working principles of most optoelectronic synapses mainly rely on physical behaviors while missing chemical-electric synaptic processes critical for mimicking biorealistic neuromorphic functionality. Herein, we report a photoelectrochemical synaptic device based on p-AlGaN/n-GaN semiconductor nanowires to incorporate chemical-electric synaptic behaviors into optoelectronic synapses, demonstrating unparalleled dual-modal plasticity and chemically-regulated neuromorphic functions through the interplay of internal photo-electric and external electrolyte-mediated chemical-electric processes. Electrical modulation by implementing closed or open-circuit enables switching of optoelectronic synaptic operation between short-term and long-term plasticity. Furthermore, inspired by transmembrane receptors that connect extracellular and intracellular events, synaptic responses can also be effectively amplified by applying chemical modifications to nanowire surfaces, which tune external and internal charge behaviors. Notably, under varied external electrolyte environments (ion/molecule species and concentrations), our device successfully mimics chemically-regulated synaptic activities and emulates intricate oxidative stress-induced biological phenomena. Essentially, we demonstrate that through the nanowire photoelectrochemical synapse configuration, optoelectronic synapses can be incorporated with chemical-electric behaviors to bridge the gap between classic optoelectronic synapses and biological synapses, providing a promising platform for multifunctional neuromorphic applications.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Liao, F. et al. Bioinspired in-sensor visual adaptation for accurate perception. Nat. Electron.5, 84–91 (2022). 10.1038/s41928-022-00713-1 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

