Induction and maintenance of mucosal healing in Crohn's disease with ustekinumab in clinical practice across all care levels in Germany (MUCUS)
- PMID: 39227642
- PMCID: PMC11371836
- DOI: 10.1038/s41598-024-70241-9
Induction and maintenance of mucosal healing in Crohn's disease with ustekinumab in clinical practice across all care levels in Germany (MUCUS)
Abstract
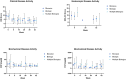
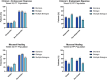
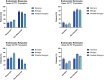
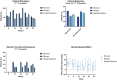
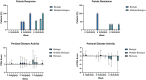
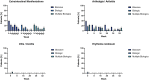
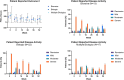
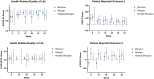
The impact of ustekinumab (UST) on mucosal- and fistula healing and extraintestinal manifestations (EIM) in Crohn's disease (CD) were not fully elucidated in the registration trials. In this prospective, multicenter study (EudraCT number: 2017-005151-83) we evaluated the German label real-world-effectiveness of UST to achieve the primary endpoint of combined clinical and endoscopic response at week 52 and several secondary endpoints. Of 79 screened we enrolled 52 patients (female n = 28, bionaïve n = 13, biologic n = 39). At week 52 (per protocol analysis), 52% (n = 13/25) of patients achieved the primary endpoint [50% (n = 3/6) in the bionaïve, 45.5% (n = 5/11) biologic, 62.5% (n = 5/8 ) multiple biologics cohorts, respectively with age as independent predictor [OR 95% CI 0.933 (0.873, 0.998) p = 0.043], 60% (n = 15/25) achieved endoscopic response [50% (n = 3/6) in the bionaïve, 54.5% (n = 6/11) biologic, 75% (n = 6/8) multiple biologics cohorts, respectively], 36% (n = 9/25) achieved endoscopic remission [50% (n = 3/6) in the bionaïve, 27.3% (n = 3/11) biologic, 37.5% (n = 3/8) multiple biologics cohorts, respectively], 48% (n = 12/25) achieved mucosal healing [50% (n = 3/6) in the bionaïve, 36.4% (n = 4/11) biologic, 62.5% (n = 5/8) multiple biologics cohorts, respectively]. All achieved a fistula response and 33.3% (n = 1/3) in the multiple biologics group fistula remission at week 52. EIM decreased (week 0 28.2% vs. week 52 8%). CRP, FCP, PRO-2, EQ-5D-5L improved throughout. 36 patients (69.2%) experienced ≥ 1 treatment emergent adverse event, in 8 (15.4%) cases rated as severe and in 5 (9.6%) leading to UST discontinuation, but no very severe events or deaths. The effectiveness of UST was better than in the registration trials.
Keywords: Crohn’s disease; Extraintestinal manifestations; Inflammatory bowel disease; Mucosal healing; Quality of life; Ustekinumab.
© 2024. The Author(s).
Conflict of interest statement
D.C.B. has over the past five years served on scientific advisory boards, speaker or moderator at CME events for, and/or received travel support from Janssen-Cilag, AbbVie, Pfizer, BMS, Falk Foundation, Takeda, Eli Lilly, Tillotts Pharma AG, and Galapagos. J.S. has received consulting fees from Abbvie, Bristol Myers Squibb, Dr Schär, Falk, Ferring, Fresenius Kabi, Immundiagnostik, Janssen, Medice, MSD, Pfizer, Pharmacosmos, Shire, Takeda, Thermofisher, Vifor, lecturing fees from Vifor Pharma, and is an Advisory Board member for Abbvie, Bristol Myers Squibb, Dr Schär, Ferring, Fresenius Kabi, Immundiagnostik, Janssen, MSD, NPS, Pharmacosmos, Takeda, Vifor and Shield. T.O. was the recipient of an unrestricted research grant from Biogen, Germany. Over the past five years T.O. has served on scientific advisory boards, and/or served as speaker or moderator at conferences and CME events for, and/or received travel support from, AbbVie, Amgen, Biogen, BMS, Celltrion, Janssen-Cilag, Lilly, MSD, Pfizer, Sandoz, Takeda, Tillots, Viatris and Vifor. A.L. has served on scientific advisory boards, and/or served as speaker or moderator for AbbVie, Biogen, BMS, Falk Foundation, Ferring, Hexal, Janssen, MSD, Nutrimmun, Pfizer, Takeda, and Tillotts Pharma. AS reports research funding from AbbVie and Celltrion. He has received lecture fees from AbbVie, Amgen, Astellas, Biogen, Celltrion, Janssen, Falk Foundation, Ferring, MSD, Recordati Pharma, Streamed-Up and Takeda and consulting fees from AbbVie, Astellas, Amgen, Biogen, CLS Behring, Consal, Eli Lilly, Galapagos, Hexal, Janssen, MSD, Norgine, Pfizer Pharma Takeda and Tillots Pharma. S.H. has received consulting and lecturing fees and has served on scientific advisory boards from Abbvie, Bristol Myers Squibb, Eli Lilly, Fresenius Kabi, Janssen, Galapagos, Pfizer, Pharmacosmos, Shield, Takeda. U.v.A. has received consulting and lecturing fees from Abbvie, Bristol Myers Squibb,EsoCap, Falk Foundation, Janssen, MSD, Pfizer, Pharmacosmos, Sanofi, Takeda, ViforPharma, and is an Advisory Board member for Abbvie, Bristol Myers Squibb, Janssen, MSD, Pharmacosmos, Takeda, Vifor , Pfizer and Sanofi. D.S., P.G. and A.F. declare no conflict of interest.
Figures
References
-
- Baumgart, D. C. Crohn's Disease and Ulcerative Colitis From Epidemiology and Immunobiology to a Rational Diagnostic and Therapeutic Approach: 10.1007/978-3-319-33703-6
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

