Effects of fructan and gluten on gut microbiota in individuals with self-reported non-celiac gluten/wheat sensitivity-a randomised controlled crossover trial
- PMID: 39227818
- PMCID: PMC11373345
- DOI: 10.1186/s12916-024-03562-1
Effects of fructan and gluten on gut microbiota in individuals with self-reported non-celiac gluten/wheat sensitivity-a randomised controlled crossover trial
Abstract
Background: Individuals with non-celiac gluten/wheat sensitivity (NCGWS) experience improvement in gastrointestinal symptoms following a gluten-free diet. Although previous results have indicated that fructo-oligosaccharides (FOS), a type of short-chain fructans, were more likely to induce symptoms than gluten in self-reported NCGWS patients, the underlying mechanisms are unresolved.
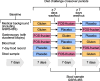
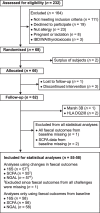
Methods: Our main objective was therefore to investigate whether FOS-fructans and gluten affect the composition and diversity of the faecal microbiota (16S rRNA gene sequencing), faecal metabolites of microbial fermentation (short-chain fatty acids [SCFA]; gas chromatography with flame ionization detector), and a faecal biomarker of gut inflammation (neutrophil gelatinase-associated lipocalin, also known as lipocalin 2, NGAL/LCN2; ELISA). In the randomised double-blind placebo-controlled crossover study, 59 participants with self-reported NCGWS underwent three different 7-day diet challenges with gluten (5.7 g/day), FOS-fructans (2.1 g/day), and placebo separately (three periods, six challenge sequences).
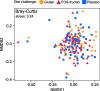
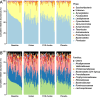
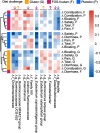
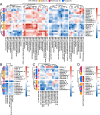
Results: The relative abundances of certain bacterial taxa were affected differently by the diet challenges. After the FOS-fructan challenge, Fusicatenibacter increased, while Eubacterium (E.) coprostanoligenes group, Anaerotruncus, and unknown Ruminococcaceae genera decreased. The gluten challenge was primarily characterized by increased abundance of Eubacterium xylanophilum group. However, no differences were found for bacterial diversity (α-diversity), overall bacterial community structure (β-diversity), faecal metabolites (SCFA), or NGAL/LCN2. Furthermore, gastrointestinal symptoms in response to FOS-fructans were generally not linked to substantial shifts in the gut bacterial community. However, the reduction in E. coprostanoligenes group following the FOS-fructan challenge was associated with increased gastrointestinal pain. Finally, correlation analysis revealed that changes in gastrointestinal symptoms following the FOS-fructan and gluten challenges were linked to varying bacterial abundances at baseline.
Conclusions: In conclusion, while FOS-fructans induced more gastrointestinal symptoms than gluten in the NCGWS patients, we did not find that substantial shifts in the composition nor function of the faecal microbiota could explain these differences in the current study. However, our results indicate that individual variations in baseline bacterial composition/function may influence the gastrointestinal symptom response to both FOS-fructans and gluten. Additionally, the change in E. coprostanoligenes group, which was associated with increased symptoms, implies that attention should be given to these bacteria in future trials investigating the impact of dietary treatments on gastrointestinal symptoms.
Trial registration: Clinicaltrials.gov as NCT02464150.
Keywords: FODMAP; Fructan; Fructo-oligosaccharides (FOS); Gastrointestinal symptoms; Gluten; Gut microbiota; Lipocalin-2 (LCN2); Neutrophil gelatinase-associated lipocalin (NGAL); Non-celiac gluten/wheat sensitivity (NCGWS); Short-chain fatty acids (SCFA).
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Henggeler JC, Veríssimo M, Ramos F. Non-coeliac gluten sensitivity: a review of the literature. Trends Food Sci Technol. 2017;66:84–92. 10.1016/j.tifs.2017.05.018 - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

