Fully automated epicardial adipose tissue volume quantification with deep learning and relationship with CAC score and micro/macrovascular complications in people living with type 2 diabetes: the multicenter EPIDIAB study
- PMID: 39227844
- PMCID: PMC11373274
- DOI: 10.1186/s12933-024-02411-y
Fully automated epicardial adipose tissue volume quantification with deep learning and relationship with CAC score and micro/macrovascular complications in people living with type 2 diabetes: the multicenter EPIDIAB study
Abstract
Background: The aim of this study (EPIDIAB) was to assess the relationship between epicardial adipose tissue (EAT) and the micro and macrovascular complications (MVC) of type 2 diabetes (T2D).
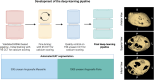
Methods: EPIDIAB is a post hoc analysis from the AngioSafe T2D study, which is a multicentric study aimed at determining the safety of antihyperglycemic drugs on retina and including patients with T2D screened for diabetic retinopathy (DR) (n = 7200) and deeply phenotyped for MVC. Patients included who had undergone cardiac CT for CAC (Coronary Artery Calcium) scoring after inclusion (n = 1253) were tested with a validated deep learning segmentation pipeline for EAT volume quantification.
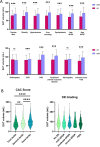
Results: Median age of the study population was 61 [54;67], with a majority of men (57%) a median duration of the disease 11 years [5;18] and a mean HbA1c of7.8 ± 1.4%. EAT was significantly associated with all traditional CV risk factors. EAT volume significantly increased with chronic kidney disease (CKD vs no CKD: 87.8 [63.5;118.6] vs 82.7 mL [58.8;110.8], p = 0.008), coronary artery disease (CAD vs no CAD: 112.2 [82.7;133.3] vs 83.8 mL [59.4;112.1], p = 0.0004, peripheral arterial disease (PAD vs no PAD: 107 [76.2;141] vs 84.6 mL[59.2; 114], p = 0.0005 and elevated CAC score (> 100 vs < 100 AU: 96.8 mL [69.1;130] vs 77.9 mL [53.8;107.7], p < 0.0001). By contrast, EAT volume was neither associated with DR, nor with peripheral neuropathy. We further evidenced a subgroup of patients with high EAT volume and a null CAC score. Interestingly, this group were more likely to be composed of young women with a high BMI, a lower duration of T2D, a lower prevalence of microvascular complications, and a higher inflammatory profile.
Conclusions: Fully-automated EAT volume quantification could provide useful information about the risk of both renal and macrovascular complications in T2D patients.
Keywords: CAC score; Cardiac computed tomography; Deep learning; Epicardial adipose tissue; Type 2 diabetes.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, et al. Pericardial Fat, Visceral Abdominal Fat, Cardiovascular Disease Risk Factors, and Vascular Calcification in a Community-Based Sample: The Framingham Heart Study. Circulation. 2008;117(5):605–13. 10.1161/CIRCULATIONAHA.107.743062 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

