Divergent effects of tumor necrosis factor (TNF) in sepsis: a meta-analysis of experimental studies
- PMID: 39227889
- PMCID: PMC11373197
- DOI: 10.1186/s13054-024-05057-0
Divergent effects of tumor necrosis factor (TNF) in sepsis: a meta-analysis of experimental studies
Abstract
Introduction: Experimental studies in animals have yielded conflicting results on the role of Tumor Necrosis Factor (TNF) in sepsis and endotoxemia, with some reporting adaptive and others inappropriate effects. A meta-analysis of the available literature was performed to determine the factors explaining this discrepancy.
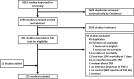
Methods: The study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The protocol was registered with PROSPERO (CRD42020167384) prior to data collection. PubMed and Embase were the databases queried. Risk of bias was evaluated using the SYRCLE Risk of Bias Tool. All animal studies investigating sepsis-related mortality and modified TNF signaling were considered eligible. The exclusion criteria were: lack of mortality data, 7-day mortality rates below 10% in both wild type and TNF-altered pathway animals, and absence of an English abstract. To determine the role of TNF according to the experimental protocol, three approaches were used: first an approach based on the statistical significance of each experiment, then the pooled mortality was calculated, and finally the weighted risk ratio for mortality was assessed.
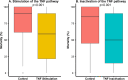
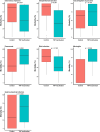
Results: A total of 175 studies were included in the analysis, comprising a total of 760 experiments and involving 19,899 animals. The main species used were mice (77%) and rats (21%). The most common method of TNF pathway modulation was TNF pathway inactivation that was primarily associated with an inappropriate secretion of TNF. At the opposite, TNF injection was associated with an adaptive role of TNF. Lipopolysaccharide (LPS) injection was the most used stimulus to establish an infectious model (42%) and was strongly associated with an inappropriate role of TNF. Conversely, live bacterial models, especially the cecal ligation and puncture (CLP) model, pneumonia, meningitis, and gastrointestinal infection, were associated with an adaptive role. This was particularly evident for Listeria monocytogenes, Streptococcus pneumoniae.
Conclusion: The role of TNF during infection varies depending on the experimental model used. Models that mimic clinical conditions, based on virulent bacteria that cause high mortality even at low inocula, demonstrated an adaptive role of TNF. Conversely, models based on LPS or low-pathogenic live bacteria, administered at doses well above physiological thresholds and combined with early antibiotic therapy, were associated with an inappropriate role.
Keywords: Experimental studies; Inactivation; LPS infection; Mortality; Pathogen; Sepsis; TNF.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Tracey KJ, Lowry SF, Fahey TJ, et al. Cachectin/tumor necrosis factor induces lethal shock and stress hormone responses in the dog. Surg Gynecol Obstet. 1987;164(5):415–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

