Strategies to enhance risk communication about medicines in Malaysia: a Delphi study among multinational experts
- PMID: 39227905
- PMCID: PMC11373486
- DOI: 10.1186/s12913-024-11476-0
Strategies to enhance risk communication about medicines in Malaysia: a Delphi study among multinational experts
Abstract
Background: Effective risk communication about medicines is crucial to the success of all pharmacovigilance activities but remains a worldwide challenge. Risk communication has been conducted in Malaysia for decades, yet awareness on the communication methods remains low among healthcare professionals. While international guidelines are available, clear guidance on effectively communicating the risks of medicines in specific countries is scarce. This study aimed to establish a consensus on the priority strategies for enhancing risk communication about medicines by regulators.
Methods: We conducted a two-round modified Delphi survey among local and international communication experts, and also recipients of medicines risk communication in Malaysia. We developed a list of 37 strategies based on the findings of our previous studies. In Round 1, participants were asked to rate the priority for each strategy using a 5-point Likert scale and suggest additional strategies via free-text comments. Strategies scoring a mean of ≥ 3.75 were included in Round 2. We defined consensus for the final list of strategies a priori as > 75% agreement. Data were analysed using descriptive statistics and thematic analysis.
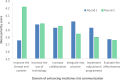
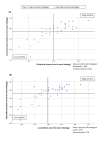
Results: Our final Delphi panel (n = 39, 93% response rate) comprised medicines communication experts from nine countries and Malaysian healthcare professionals. Following Round 1, we dropped 14 strategies and added 11 strategies proposed by panellists. In the second round, 21 strategies achieved consensus. The priority areas identified were to improve the format and content of risk communication, increase the use of technology, and increase collaboration with various stakeholders. Priority ratings for the strategy "to offer incentives to pharmaceutical companies which maintain effective communication systems" were significantly higher among recipients compared to communicators [χ2(1, N = 39) = 10.1; p = 0.039] and among local versus international panellists [χ2(1, N = 39) = 14.3; p = 0.007].
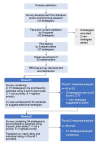
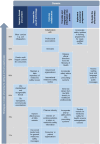
Conclusions: Our study identified 21 priority strategies, which were used to develop a strategic plan for enhancing medicines risk communication. This plan is potentially adaptable to all countries with developing pharmacovigilance systems. The difference in views between communicators and recipients, as well as local and international panellists, highlights the importance of involving multiple stakeholders in research.
Keywords: Consensus; Delphi survey; Developing country; Effective communication; Pharmacovigilance; Safety information.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Bahri P, Bowring G, Edwards BD, Anton C, Aronson JK, Caro-Rojas A, et al. Communicating for the safe use of Medicines: progress and directions for the 2020s promoted by the Special Interest Group of the International Society of Pharmacovigilance. Drug Saf. 2023. 10.1007/s40264-023-01285-5. 10.1007/s40264-023-01285-5 - DOI - PMC - PubMed
-
- Bahri P, Dodoo AN, Edwards BD, Edwards IR, Fermont I, Hagemann U, et al. The ISoP CommSIG for improving Medicinal product risk communication: a New Special Interest Group of the International Society of Pharmacovigilance. Drug Saf. 2015. 10.1007/s40264-015-0301-0. 10.1007/s40264-015-0301-0 - DOI - PubMed
-
- Bahri P, Harrison-Woolrych M. How to improve communication for the safe use of Medicines? Discussions on Social Marketing and patient-tailored approaches at the annual meetings of the WHO Programme for International Drug Monitoring. Drug Saf. 2012. 10.1007/BF03261993. 10.1007/BF03261993 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

