The antifibrotic potential of IMT504: modulation of GLAST + Wnt1 + bone marrow stromal progenitors and hepatic microenvironment
- PMID: 39227908
- PMCID: PMC11373403
- DOI: 10.1186/s13287-024-03896-w
The antifibrotic potential of IMT504: modulation of GLAST + Wnt1 + bone marrow stromal progenitors and hepatic microenvironment
Abstract
Background: The immunomodulatory oligodeoxynucleotide (ODN) IMT504 might harbor antifibrotic properties within the liver.
Methods: Fibrosis models were induced in mice through thioacetamide (TAA) administration and bile-duct ligation. Cre-loxP mice were utilized to identify GLAST + Wnt1 + bone marrow stromal progenitors (BMSPs) and to examine their contribution with cells in the liver. In vivo and in vitro assays; flow-cytometry, immunohistochemistry, and qPCR were conducted.
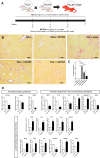
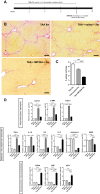
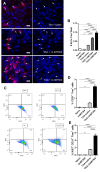
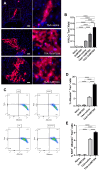
Results: IMT504 demonstrated significant inhibition of liver fibrogenesis progression and reversal of established fibrosis. Early responses to IMT504 involved the suppression of profibrogenic and proinflammatory markers, coupled with an augmentation of hepatocyte proliferation. Additionally, this ODN stimulated the proliferation and mobilization of GLAST + Wnt1 + BMSPs, likely amplifying their contribution with endothelial- and hepatocytes-like cells. Moreover, IMT504 significantly modulated the expression levels of Wnt ligands and signaling pathway/target genes specifically within GLAST + Wnt1 + BMSPs, with minimal impact on other BMSPs. Intriguingly, both IMT504 and conditioned media from IMT504-pre-treated GLAST + Wnt1 + BMSPs shifted the phenotype of fibrotic macrophages, hepatic stellate cells, and hepatocytes, consistent with the potent antifibrotic effects observed.
Conclusion: In summary, our findings identify IMT504 as a promising candidate molecule with potent antifibrotic properties, operating through both direct and indirect mechanisms, including the activation of GLAST + Wnt1 + BMSPs.
Keywords: Bone marrow stromal progenitors; GLAST; Hepatic stellate cells; Hepatocytes; Immunomodulation; Liver progenitors; Macrophages; Plasticity; Wnt.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Fiore EJ, Bayo JM, Garcia MG, Malvicini M, Lloyd R, Piccioni F, Rizzo M, Peixoto E, Sola MB, Atorrasagasti C, Alaniz L, Camilletti MA, Enguita M, Prieto J, Aquino JB, Mazzolini G. Mesenchymal stromal cells engineered to produce IGF-I by recombinant adenovirus ameliorate liver fibrosis in mice. Stem Cells Dev. 2015;24(6):791–801. 10.1089/scd.2014.0174. 10.1089/scd.2014.0174 - DOI - PMC - PubMed
-
- Baccin C, Al-Sabah J, Velten L, Helbling PM, Grunschlager F, Hernandez-Malmierca P, Nombela-Arrieta C, Steinmetz LM, Trumpp A, Haas S. Combined single-cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization. Nat Cell Biol. 2020;22(1):38–48. 10.1038/s41556-019-0439-6. 10.1038/s41556-019-0439-6 - DOI - PMC - PubMed
-
- Baryawno N, Przybylski D, Kowalczyk MS, Kfoury Y, Severe N, Gustafsson K, Kokkaliaris KD, Mercier F, Tabaka M, Hofree M, Dionne D, Papazian A, Lee D, Ashenberg O, Subramanian A, Vaishnav ED, Rozenblatt-Rosen O, Regev A, Scadden DT. A Cellular Taxonomy of the bone marrow stroma in Homeostasis and Leukemia. Cell. 2019;177(7):1915–32. 10.1016/j.cell.2019.04.040. e1916. 10.1016/j.cell.2019.04.040 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

