MiR-100-5p-rich small extracellular vesicles from activated neuron to aggravate microglial activation and neuronal activity after stroke
- PMID: 39227960
- PMCID: PMC11370036
- DOI: 10.1186/s12951-024-02782-0
MiR-100-5p-rich small extracellular vesicles from activated neuron to aggravate microglial activation and neuronal activity after stroke
Abstract
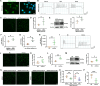
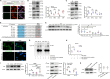
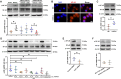
Ischemic stroke is a common cause of mortality and severe disability in human and currently lacks effective treatment. Neuronal activation and neuroinflammation are the major two causes of neuronal damage. However, little is known about the connection of these two phenomena. This study uses middle cerebral artery occlusion mouse model and chemogenetic techniques to study the underlying mechanisms of neuronal excitotoxicity and severe neuroinflammation after ischemic stroke. Chemogenetic inhibition of neuronal activity in ipsilesional M1 alleviates infarct area and neuroinflammation, and improves motor recovery in ischemia mice. This study identifies that ischemic challenge triggers neuron to produce unique small extracellular vesicles (EVs) to aberrantly activate adjacent neurons which enlarge the neuron damage range. Importantly, these EVs also drive microglia activation to exacerbate neuroinflammation. Mechanistically, EVs from ischemia-evoked neuronal activity induce neuronal apoptosis and innate immune responses by transferring higher miR-100-5p to adjacent neuron and microglia. MiR-100-5p can bind to and activate TLR7 through U18U19G20-motif, thereby activating NF-κB pathway. Furthermore, knock-down of miR-100-5p expression improves poststroke outcomes in mice. Taken together, this study suggests that the combination of inhibiting aberrant neuronal activity and the secretion of specific EVs-miRNAs may serve as novel methods for stroke treatment.
Keywords: MiR-100-5p; Microglial activation; Neuronal activity; Small extracellular vesicles; Stroke.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Hossmann KA. Periinfarct depolarizations. Cerebrovasc Brain Metab Rev. 1996;8:195–208. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

