Intrinsic ADRB2 inhibition improves CAR-T cell therapy efficacy against prostate cancer
- PMID: 39228124
- PMCID: PMC11489547
- DOI: 10.1016/j.ymthe.2024.08.028
Intrinsic ADRB2 inhibition improves CAR-T cell therapy efficacy against prostate cancer
Abstract
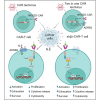
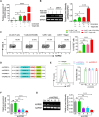
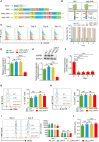
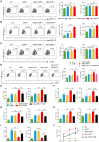
Chimeric antigen receptor (CAR)-T cell therapy has shown limited success in patients with solid tumors. Recent in vitro and in vivo data have shown that adrenoceptor beta-2 (ADRB2) is a novel checkpoint receptor that inhibits T cell-mediated anti-tumor responses. To inhibit ADRB2-mediated inhibitory signaling, we downregulated ADRB2 in CAR-T (shβ2-CAR-T) cells via RNA interference, assessed different parameters, and compared them with conventional second-generation CAR-T cells. ADRB2 knockdown CAR-T cells exhibited enhanced cytotoxicity against prostate cancer cell lines in vitro, by increasing CD69, CD107a, GzmB, IFN-γ, T-bet, and GLUT-1. In addition, ADRB2 deficiency led to improved proliferation, increased CD8/CD4 T cell ratio, and decreased apoptosis in CAR-T cells. shβ2-CAR-T cells expressed more Bcl-2 and led to the generation of more significant proportions of T central memory cells. Finally, the ZAP-70/NF-κB signaling axis was shown to be responsible for the improved functions of novel CAR-T cells. In tumor-bearing mice, shβ2-CAR-T cells performed better than conventional CAR-T cells in eradicating prostate tumors. The study provides the basis for future clinical and translational CAR-T cell research to focus on adrenergic stress-mediated challenges in the tumor microenvironment of stressed tumors.
Keywords: ADRB2; CAR; CAR-T cell therapy; adrenergic stress; adrenoceptor beta-2; chimeric antigen receptor; combination therapy; immune checkpoint; immunotherapy; neuroimmunology; prostate cancer; tumor microenvironment.
Copyright © 2024 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

