Implant-based Breast Reconstruction Salvage with Negative Pressure Wound Therapy with Instillation: An Evaluation of Outcomes
- PMID: 39228420
- PMCID: PMC11368219
- DOI: 10.1097/GOX.0000000000006116
Implant-based Breast Reconstruction Salvage with Negative Pressure Wound Therapy with Instillation: An Evaluation of Outcomes
Abstract
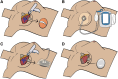
Background: Implant infection is problematic in breast reconstruction. Traditionally, infected tissue expanders (TE)/implants are removed for several months before replacement, resulting in breast reconstruction delay. Salvage involving device removal, negative pressure wound therapy with instillation and dwell (NPWTi-d) placement, and early staged TE/implant replacement within a few days has been described. The purpose of this study was to compare outcomes of the NPWTi-d salvage pathway with traditional implant removal.
Methods: A retrospective review was performed on patients who underwent implant-based reconstruction and developed TE/implant infection/exposure requiring removal. Patients were divided into two groups. Group 1 had TE/implant removal, NPWTi-d placement, and TE/implant replacement 1-4 days later. Group 2 (control) underwent standard TE/implant removal and no NPWTi-d. Reinfection after TE/implant salvage, TE/implant-free days, and time to final reconstruction were assessed.
Results: The study included 47 patients (76 TE/implants) in group 1 (13 patients, 16 TE/implants) and group 2 (34 patients, 60 TE/implants). The success rate (no surgical-site infection within 90 days) of implant salvage was 81.3% in group 1. No group 1 patients abandoned completing reconstruction after TE/implant loss versus 38.2% (13 of 34) in group 2 (P = 0.0094). Mean implant-free days was 2.5 ± 1.2 in group 1 versus 134.6 ± 78.5 in group 2 (P = 0.0001). The interval to final implant-based reconstruction was 69.0 ± 69.7 days in group 1 versus 225.6 ± 93.6 days in group 2 (P = 0.0001).
Conclusions: A breast implant salvage pathway with infected device removal, NPWTi-d placement, and early TE/implant replacement was successful in 81.3%. Patients experienced 132 less implant-free days and faster time to final reconstruction.
Copyright © 2024 The Authors. Published by Wolters Kluwer Health, Inc. on behalf of The American Society of Plastic Surgeons.
Conflict of interest statement
The authors have no financial interest to declare in relation to the content of this article.
Figures
References
-
- Nahabedian MY, Tsangaris T, Momen B, et al. Infectious complications following breast reconstruction with expanders and implants. Plast Reconstr Surg. 2003;112:467–476. - PubMed
-
- Dassoulas KR, Wang J, Thuman J, et al. Reducing infection rates in implant-based breast reconstruction: impact of an evidence-based protocol. Ann Plast Surg. 2018;80:493–499. - PubMed
-
- Brand KG. Infection of mammary prostheses: a survey and the question of prevention. Ann Plast Surg. 1993;30:289–295. - PubMed
LinkOut - more resources
Full Text Sources
