Post-vaccine immune status surveillance of Covishield vaccine and associated AEFI in previously SARS-Cov-2 infected seropositive and seronegative population in Assam
- PMID: 39228552
- PMCID: PMC11368280
- DOI: 10.4103/jfmpc.jfmpc_169_24
Post-vaccine immune status surveillance of Covishield vaccine and associated AEFI in previously SARS-Cov-2 infected seropositive and seronegative population in Assam
Abstract
Background: COVID-19 an infectious disease caused by the SARS-CoV-2 virus, started in late 2019 and became a pandemic within a short period. To respond to the pandemic vaccines like Covishield, Covaxin, Sputnik V, Covovax, etc., were developed rapidly. However, there were raising concerns about the development of immunity as well as adverse events following vaccination.
Objectives: To compare anti-SARS-CoV-2 IgG antibody titres at different time-points post-vaccination between baseline seropositive and seronegative groups and to assess the adverse events following the 1st dose of Covishield vaccine among adult beneficiaries attending vaccination centre in a tertiary care hospital of Upper Assam.
Materials and methods: Prospective Cohort study was conducted from July 2021 to June 2022 among adult beneficiaries receiving the Covishield vaccine. The oral questionnaire was used incorporating socio-demographic variables, and clinical profiles including co-morbidities and adverse events following vaccination. Data analysis was done by Microsoft Excel and SPSS.
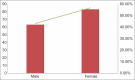
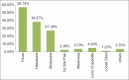
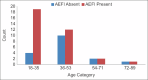
Results: Out of a total of 146 study participants, IgG estimation showed 61% as seropositive and the rest as seronegative. A total of 55.40% had minor adverse events, majority of them were females (53.08%) and 88.80% belonged to 18-59 years compared to 11.11% above 60 years of age. The majority (71.60%) did not have any co-morbidities and the major AEFI was NIL among the study participants. The study group had 61% seropositive previously infected.
Conclusion: Covishield vaccination induces an immune response and 90% seroconversion is achieved after 1st dose (booster dose). Antibody titres of the seropositive group by natural infection of SARS-CoV-2 were higher than seronegative cohort seroconverted by vaccination. The AEFI observed were minor and can be commented as safer.
Keywords: AEFI; Covishield; SARS-CoV-2; co-morbidities; serosurveillance.
Copyright: © 2024 Journal of Family Medicine and Primary Care.
Conflict of interest statement
There are no conflicts of interest.
Figures


References
-
- Worldometer COVID-19 Coronavirus Pandemic. 2021, 2022a. 2023. [[Last accessed on 2024 Apr 14]]. Available from: https://www.worldometers. info/coronavirus/
-
- Kulkarni PS, Padmapriyadarsini C, Vekemans J, Bavdekar A, Gupta M, Kulkarni P, et al. A phase 2/3, participant-blind, observer-blind, randomised, controlled study to assess the safety and immunogenicity of SII-ChAdOx1 nCoV-19 (COVID-19 vaccine) in adults in India. EClinicalMedicine. 2021;42:10218. doi: 10.1016/j.eclinm.2021.101218. - PMC - PubMed
-
- Drugs Controller General of India. Press Statement by the Drugs Controller General of India (DCGI) on Restricted Emergency approval of COVID-19 virus vaccine. 2021. (https://www.icmr.gov.in/pdf/press_realease_files/HFW_DCGI_energency_use_...
-
- World Health Organization. COVID-19 vaccines: Knowledge gaps and research priorities WHO ad hoc consultation. 2021. (https://www.who.int/publications/m/item/covid-19-vaccines-knowledge-gaps...
LinkOut - more resources
Full Text Sources
Miscellaneous
