Reiterated male-to-female violence disrupts hippocampal estrogen receptor β expression, prompting anxiety-like behavior
- PMID: 39228787
- PMCID: PMC11369378
- DOI: 10.1016/j.isci.2024.110585
Reiterated male-to-female violence disrupts hippocampal estrogen receptor β expression, prompting anxiety-like behavior
Abstract
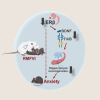
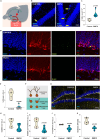
Intimate partner violence (IPV) is a significant public health concern whose neurological/behavioral sequelae remain to be mechanistically explained. Using a mouse model recapitulating an IPV scenario, we evaluated the female brain neuroendocrine alterations produced by a reiterated male-to-female violent interaction (RMFVI). RMFVI prompted anxiety-like behavior in female mice whose hippocampus displayed a marked neuronal loss and hampered neurogenesis, namely reduced BrdU-DCX-positive nuclei and diminished dendritic arborization in the dentate gyrus (DG): effects paralleled by a substantial downregulation of the estrogen receptor β (ERβ). After RMFVI, the DG harbored reduced brain-derived neurotrophic factor (BDNF) pools and tyrosine kinase receptor B (TrkB) phosphorylation. Accordingly, ERβ knockout (KO) mice had heightened anxiety and curtailed BDNF levels at baseline while dying prematurely during the RMFVI procedure. Strikingly, injecting an ERβ antagonist or agonist into the wild-type (WT) female hippocampus enhanced or reduced anxiety, respectively. Thus, reiterated male-to-female violence jeopardizes hippocampal homeostasis, perturbing the ERβ/BDNF axis and ultimately instigating anxiety and chronic stress.
Keywords: Behavioral neuroscience; Molecular neuroscience; Neuroscience.
© 2024 Published by Elsevier Inc.
Conflict of interest statement
The authors declare no conflicts of interests.
Figures




References
-
- Oram S., Fisher H.L., Minnis H., Seedat S., Walby S., Hegarty K., Rouf K., Angénieux C., Callard F., Chandra P.S., et al. The Lancet Psychiatry Commission on intimate partner violence and mental health: advancing mental health services, research, and policy. Lancet Psychiatr. 2022;9:487–524. doi: 10.1016/S2215-0366(22)00008-6. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

