This is a preprint.
Lipid- and protein-directed photosensitizer proximity labeling captures the cholesterol interactome
- PMID: 39229057
- PMCID: PMC11370482
- DOI: 10.1101/2024.08.20.608660
Lipid- and protein-directed photosensitizer proximity labeling captures the cholesterol interactome
Abstract
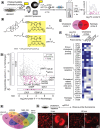
The physical properties of cellular membranes, including fluidity and function, are influenced by protein and lipid interactions. In situ labeling chemistries, most notably proximity-labeling interactomics are well suited to characterize these dynamic and often fleeting interactions. Established methods require distinct chemistries for proteins and lipids, which limits the scope of such studies. Here we establish a singlet-oxygen-based photocatalytic proximity labeling platform (POCA) that reports intracellular interactomes for both proteins and lipids with tight spatiotemporal resolution using cell-penetrant photosensitizer reagents. Using both physiologically relevant lipoprotein-complexed probe delivery and genetic manipulation of cellular cholesterol handling machinery, cholesterol-directed POCA captured established and unprecedented cholesterol binding proteins, including protein complexes sensitive to intracellular cholesterol levels and proteins uniquely captured by lipoprotein uptake. Protein-directed POCA accurately mapped known intracellular membrane complexes, defined sterol-dependent changes to the non-vesicular cholesterol transport protein interactome, and captured state-dependent changes in the interactome of the cholesterol transport protein Aster-B. More broadly, we find that POCA is a versatile interactomics platform that is straightforward to implement, using the readily available HaloTag system, and fulfills unmet needs in intracellular singlet oxygen-based proximity labeling proteomics. Thus, we expect widespread utility for POCA across a range of interactome applications, spanning imaging to proteomics.
Conflict of interest statement
Ethics declarations The authors declare no known conflicts of interest.
Figures




References
-
- Singer S. J. & Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science 175, 720–731 (1972). - PubMed
-
- Simons K. & van Meer G. Lipid sorting in epithelial cells. Biochemistry 27, 6197–6202 (1988). - PubMed
-
- Wang H., Chen J., Hollister K., Sowers L. C. & Forman B. M. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol. Cell 3, 543–553 (1999). - PubMed
-
- Breslowa J. L., Lothrop D. A., Spaulding D. R. & Kandutsch A. A. Cholesterol, 7-ketocholesterol and 25-hydroxycholesterol uptake studies and effect on 3-hydroxy-3-methylglutaryl-coenzyme a reductase activity in human fibroblasts. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism 398, 10–17 (1975). - PubMed
-
- Ohashi R., Mu H., Wang X., Yao Q. & Chen C. Reverse cholesterol transport and cholesterol efflux in atherosclerosis. QJM 98, 845–856 (2005). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
