This is a preprint.
Non-canonical Wnt signaling triggered by WNT2B drives adrenal aldosterone production
- PMID: 39229119
- PMCID: PMC11370552
- DOI: 10.1101/2024.08.23.609423
Non-canonical Wnt signaling triggered by WNT2B drives adrenal aldosterone production
Abstract
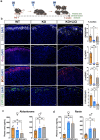
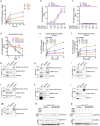
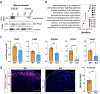
The steroid hormone aldosterone, produced by the zona glomerulosa (zG) of the adrenal gland, is a master regulator of plasma electrolytes and blood pressure. While aldosterone control by the renin-angiotensin system is well understood, other key regulatory factors have remained elusive. Here, we replicated a prior association between a non-coding variant in WNT2B and an increased risk of primary aldosteronism, a prevalent and debilitating disease caused by excessive aldosterone production. We further show that in both mice and humans, WNT2B is expressed in the mesenchymal capsule surrounding the adrenal cortex, in close proximity to the zG. Global loss of Wnt2b in the mouse results in a dysmorphic and hypocellular zG, with impaired aldosterone production. Similarly, humans harboring WNT2B loss-of-function mutations develop a novel form of Familial Hyperreninemic Hypoaldosteronism, designated here as Type 4. Additionally, we demonstrate that WNT2B signals by activating the non-canonical Wnt/planar cell polarity pathway. Our findings identify WNT2B as a key regulator of zG function and aldosterone production with important clinical implications.
Keywords: Familial Hyperreninemic Hypoaldosteronism; WNT2B; Wnt/PCP pathway; adrenal cortex; beta-catenin-independent signaling; hypoaldosteronism; non-canonical Wnt signaling; primary aldosteronism; rosette; zona glomerulosa.
Figures



References
-
- Zelander T. The ultrastructure of the adrenal cortex of the mouse. Zeitschrift für Zellforschung und Mikroskopische Anatomie. 1957;46(6):710–716. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
