Impact of E. muris infection on B. burgdorferi- induced joint pathology in mice
- PMID: 39229265
- PMCID: PMC11368855
- DOI: 10.3389/fimmu.2024.1430419
Impact of E. muris infection on B. burgdorferi- induced joint pathology in mice
Abstract
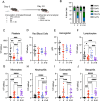
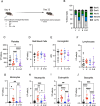
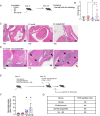
Tick-borne infections are increasing in the United States and around the world. The most common tick-borne disease in the United States is Lyme disease caused by infection with the spirochete Borrelia burgdorferi (Bb), and pathogenesis varies from subclinical to severe. Bb infection is transmitted by Ixodes ticks, which can carry multiple other microbial pathogens, including Ehrlichia species. To address how the simultaneous inoculation of a distinct pathogen impacted the course of Bb-induced disease, we used C57BL/6 (B6) mice which are susceptible to Bb infection but develop only mild joint pathology. While infection of B6 mice with Bb alone resulted in minimal inflammatory responses, mice co-infected with both Bb and the obligate intracellular pathogen Ehrlichia muris (Em) displayed hematologic changes, inflammatory cytokine production, and emergency myelopoiesis similar to what was observed in mice infected only with Em. Moreover, infection of B6 mice with Bb alone resulted in no detectable joint inflammation, whereas mice co-infected with both Em and Bb exhibited significant inflammation of the ankle joint. Our findings support the concept that co-infection with Ehrlichia can exacerbate inflammation, resulting in more severe Bb-induced disease.
Keywords: Borrelia (Borreliella) burgdorferi; Ehrlichia; hematopoiesis; infection; inflammation.
Copyright © 2024 Bonin, Torres, Marcinkiewicz, Duhamel, Yang, Pal, DiSpirito, Nowak, Lin and MacNamara.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

