Unraveling the Peroxidase Activity in Peroxiredoxins: A Comprehensive Review of Mechanisms, Functions, and Biological Significance
- PMID: 39229430
- PMCID: PMC11370188
- DOI: 10.7759/cureus.66117
Unraveling the Peroxidase Activity in Peroxiredoxins: A Comprehensive Review of Mechanisms, Functions, and Biological Significance
Abstract
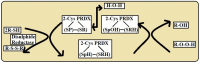
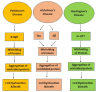
Peroxiredoxins (Prxs) are members of the antioxidant enzymes necessary for every living object in the three domains of life and play critical roles in controlling peroxide levels in cells. This comprehensive literature review aims to elucidate the peroxidase activity of Prxs, examining their roles and significance for organisms across various taxa. Ironically, the primary role of the Prxs is the peroxidase activity, which comprises the reduction of hydrogen peroxide and other organic hydroperoxides and decreases the risk of oxidative damage in the cells. The above enzymatic activity occurs through the reversible oxidation-reduction catalyzed by cysteine residues in the active site by forming sulfenic acid and reduction by intracellular reductants. Structurally and functionally, Prxs function as dimers or decamers and show different catalytic patterns according to their subfamilies or cellular compartments. Compared to the mechanisms of the other two subgroups of Prxs, including 2-Cys Prxs and atypical Prxs, the 1-Cys Prxs have monomer-dimer switch folding coupled with catalytic activity. In addition to their peroxidase activity, which is widely known, Prxs are becoming acknowledged to be involved in other signaling processes, including redox signaling and apoptosis. This aversion to oxidative stress and regulation by the cellular redox state places them at the heart of adaptive cellular responses to changes in the environment or manifestations of diseases. In conclusion, based on the data obtained and on furthering the knowledge of Prxs' structure and function, these enzymes may be classified as a diverse yet essential family of proteins that can effectively protect cells from the adverse effects of oxidative stress due to peroxidase activity. This indicates secondary interactions, summarized as peroxide detoxification or regulatory signaling, and identifies their applicability in multiple biological pathways. Such knowledge is valuable for enhancing the general comprehension of essential cellular functions and disclosing further therapeutic approaches to the diseases caused by the increased production of reactive oxygen species.
Keywords: oxidative stress; pathways; peroxidase; peroxiredoxins; redox balance.
Copyright © 2024, Qausain et al.
Conflict of interest statement
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures




References
-
- Peroxiredoxins, oxidative stress, and cell proliferation. Immenschuh S, Baumgart-Vogt E. Antioxid Redox Signal. 2005;7:768–777. - PubMed
-
- The role of thioredoxin/peroxiredoxin in the β-cell defense against oxidative damage. Stancill JS, Corbett JA. https://www.frontiersin.org/journals/endocrinology/articles/10.3389/fend... Front Endocrinol (Lausanne) 2021;12:718235. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
