PPAR gamma and PGC-1alpha activators protect against diabetic nephropathy by suppressing the inflammation and NF-kappaB activation
- PMID: 39229715
- PMCID: PMC11579552
- DOI: 10.1111/nep.14381
PPAR gamma and PGC-1alpha activators protect against diabetic nephropathy by suppressing the inflammation and NF-kappaB activation
Abstract
Aim: Inflammation plays a critical role in the progression of diabetic nephropathy. Peroxisome proliferator-activated receptor gamma (PPARγ) and its coactivator PPARγ coactivator-1 alpha (PGC-1α) enhance mitochondrial biogenesis and cellular energy metabolism but inhibit inflammation. However, the molecular mechanism through which these two proteins cooperate in the kidney remains unclear. The aim of the present study was to investigate this mechanism.
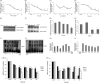
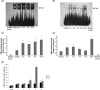
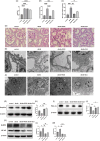
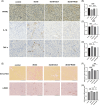
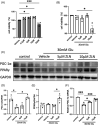
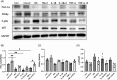
Methods: HK-2 human proximal tubular cells were stimulated by inflammatory factors, the expression of PPARγ and PGC-1α were determined via reverse transcription-quantitative polymerase chain reaction (PCR) and western blotting (WB), and DNA binding capacity was measured by an EMSA. Furthermore, db/db mice were used to establish a diabetic nephropathy model and were administered PPARγ and PGC-1α activators. Kidney injury was evaluated microscopically, and the inflammatory response was assessed via WB, immunohistochemistry and immunofluorescence staining. Besides, HK-2 cells were stimulated by high glucose and inflammatory factors with and without ZLN005 treatment, the expression of PPARγ, PGC-1α, p-p65 and p65 were determined via qPCR and WB.
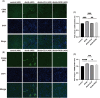
Results: Our results revealed that both TNF-α and IL-1β significantly decreased PPARγ and PGC-1 expression in vitro. Cytokines obviously decreased PPARγ DNA binding capacity. Moreover, we detected rapid activation of the NF-κB pathway in the presence of TNF-α or IL-1β. PPARγ and PGC-1α activators effectively protected against diabetic nephropathy and suppressed NF-κB expression both in db/db mice and HK-2 cells.
Conclusion: PPARγ and its coactivator PGC-1α actively participate in protecting against renal inflammation by regulating the NF-κB pathway, which highlights their potential as therapeutic targets for renal diseases.
Keywords: NF‐κB; PGC‐1α; PPARγ; inflammation; kidney.
© 2024 The Author(s). Nephrology published by John Wiley & Sons Australia, Ltd on behalf of Asian Pacific Society of Nephrology.
Conflict of interest statement
All the authors declared no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

