The INSIGHT study: a randomized, Phase III study of ripretinib versus sunitinib for advanced gastrointestinal stromal tumor with KIT exon 11 + 17/18 mutations
- PMID: 39229786
- PMCID: PMC11497949
- DOI: 10.1080/14796694.2024.2376521
The INSIGHT study: a randomized, Phase III study of ripretinib versus sunitinib for advanced gastrointestinal stromal tumor with KIT exon 11 + 17/18 mutations
Abstract
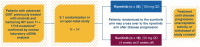
Somatic KIT activating mutations drive most gastrointestinal stromal tumors (GISTs). Disease progression eventually develops with first-line imatinib, commonly due to KIT secondary mutations, and different kinase inhibitors have various levels of treatment efficacy dependent on specific acquired resistance mutations. Ripretinib is a broad-spectrum switch-control KIT/PDGFRA tyrosine kinase inhibitor for patients with advanced GIST who received prior treatment with three or more kinase inhibitors, including imatinib. Exploratory baseline circulating tumor DNA analysis from the second-line INTRIGUE trial determined that patients with advanced GIST previously treated with imatinib harboring primary KIT exon 11 mutations and secondary resistance mutations restricted to KIT exons 17/18 had greater clinical benefit with ripretinib versus sunitinib. We describe the rationale and design of INSIGHT (NCT05734105), an ongoing Phase III open-label study of ripretinib versus sunitinib in patients with advanced GIST previously treated with imatinib exclusively harboring KIT exon 11 + 17/18 mutations detected by circulating tumor DNA.Clinical Trial Registration: NCT05734105 (ClinicalTrials.gov).
Keywords: INSIGHT; KIT mutations; Phase III trial; ctDNA mutational analysis; gastrointestinal stromal tumor; predictive marker; ripretinib; sunitinib; targeted therapy; tyrosine kinase inhibitor.
Plain language summary
Gastrointestinal stromal tumor (GIST) is rare, but it is the most common mesenchymal tumor (a type of tumor that develops from cells which give rise to soft tissues) of the gastrointestinal tract. The primary treatment for advanced GIST is medication that targets the abnormal mechanisms in cancer cells in order to block tumor growth and spread. Ripretinib is an inhibitor of a protein known as KIT, which is a member of the tyrosine kinase protein family and is involved in the growth of GIST. In a Phase III clinical trial called INTRIGUE, the effects of ripretinib and another receptor tyrosine kinase inhibitor, sunitinib, were compared in patients with advanced GIST previously treated with the drug imatinib. An exploratory analysis from the INTRIGUE trial that characterized baseline circulating tumor DNA in the blood showed a greater clinical benefit with ripretinib versus sunitinib in patients with gene mutations solely occurring in KIT exon 11 + 17 and/or 18 (exon 11 + 17/18). This article describes the rationale and design for a Phase III clinical trial called INSIGHT that will evaluate the benefit of ripretinib compared with sunitinib in patients with advanced GIST whose tumors have mutations in KIT exon 11 and KIT exon 17 and/or 18. Patients will receive ripretinib or sunitinib in 6-week cycles, and investigators will assess survival without cancer progression as the primary outcome, and overall survival, and response of the tumor to these two drugs as secondary outcomes.
Conflict of interest statement
S George reports stock/other ownership interests from Abbott Laboratories and Pfizer; honoraria from CStone Pharmaceuticals; consulting or advisory roles for Blueprint Medicines, Daiichi Sankyo, Immunicum, Kayothera, Lilly, MORE Health, Research To Practice, UpToDate, and Deciphera Pharmaceuticals, LLC; research funding from BioAtla (Inst), Blueprint Medicines (Inst), Daiichi Sankyo (Inst), Eisai (Inst), IDRX (Inst), Merck (Inst), NewBay (Inst), SpringWorks Therapeutics (Inst), Theseus Pharmaceuticals (Inst), TRACON Pharmaceuticals (Inst), and Deciphera Pharmaceuticals, LLC (Inst); patents/royalties/other intellectual property from UpToDate; and other relationships with WCG. J-Y Blay reports a leadership role with Innate Pharma; honoraria from AstraZeneca, Bayer, Bristol Myers Squibb, Ignyta, Merck Sharp & Dohme, PharmaMar, Roche, and Deciphera Pharmaceuticals, LLC; consulting/advisory roles with Bayer, Blueprint Medicines, Karyopharm Therapeutics, PharmaMar, Roche, and Deciphera Pharmaceuticals, LLC; research funding from AstraZeneca (Inst), Bayer (Inst), Bristol Myers Squibb (Inst), GlaxoSmithKline (Inst), Merck Sharp & Dohme (Inst), Novartis (Inst), OSE Immunotherapeutics (Inst), PharmaMar (Inst), Roche (Inst), and Deciphera Pharmaceuticals, LLC (Inst); and funding for travel/accommodations/expenses from Roche. P Chi reports consulting/advisory roles for NewBay Pharma, Zai Lab, and Deciphera Pharmaceuticals, LLC; patents/royalties/other intellectual property from ORIC Pharmaceuticals (immediate family member); stock/other ownership interests in ORIC Pharmaceuticals (immediate family member); and research funding from NewBay Pharma (Inst), Pfizer (Inst), and Deciphera Pharmaceuticals, LLC (Inst). RL Jones reports consulting/advisory roles for Adaptimmune Therapeutics, Athenex, Bayer, Blueprint Medicines, Boehringer Ingelheim, Clinigen Group, Daiichi Sankyo, Eisai, Epizyme, Immodulon Therapeutics, Immune Design, Karma Oncology, Lilly, Merck, Morphotek, Mundipharma, PharmaMar, Immunicum, SpringWorks Therapeutics, SynOx, TRACON Pharmaceuticals, UpToDate, and Deciphera Pharmaceuticals, LLC; funding for travel/accommodations/expenses from PharmaMar; and research funding from GlaxoSmithKline (Inst). C Serrano reports consulting/advisory roles for Blueprint Medicines, Cogent Biosciences, IDRX, Immunicum, NewBay, and Deciphera, Pharmaceuticals, LLC; funding for travel/accommodations/expenses from Bayer, Gilead Sciences, Inc., Pfizer, and PharmaMar; honoraria from PharmaMar, Roche, and Deciphera Pharmaceuticals, LLC; and research funding from IDRx (Inst) and Karyopharm Therapeutics (Inst). N Somaiah reports consulting/advisory roles for AADI Bioscience, Bayer, Blueprint Medicines, Boehringer Ingelheim, Epizyme, and Deciphera Pharmaceuticals, LLC; stock/other ownership interests (immediate family member) in Johnson & Johnson, Natera, and Pfizer; and research funding from Ascentage Pharma, GlaxoSmithKline, Karyopharm Therapeutics, Daiichi Sankyo/Lilly, AstraZeneca/MedImmune (Inst), and Deciphera Pharmaceuticals, LLC. H Gelderblom reports institutional research funding from Abbisko, AmMax Bio, Bayer, Blueprint Medicines, Daiichi Sankyo, Ipsen, Novartis, Pfizer, and Deciphera Pharmaceuticals, LLC. JR Zalcberg reports a leadership role with ICON Group; stock/other ownership interests in Amarin Corporation, BioMarin, Concert Pharmaceuticals, Frequency Therapeutics, Gilead Sciences, Madrigal Pharmaceuticals, Moderna Therapeutics, Novavax, Opthea, Orphazyme, Twist Bioscience, UniQure, and Zogenix; honoraria from Gilead Sciences, Halozyme, Merck Serono, Specialised Therapeutics, Targovax, and Deciphera Pharmaceuticals, LLC; consulting/advisory roles with 1Globe Health Institute, Center for Emerging & Neglected Diseases (CEND), FivePHusion, Genor BioPharma, Halozyme, Lipotek, Merck Serono, Merck Sharp & Dohme, Novotech, REVOLUTION MEDICINE, Specialised Therapeutics, Targovax, and Deciphera Pharmaceuticals, LLC; research funding from AstraZeneca (Inst), Bristol Myers Squibb (Inst), Eisai (Inst), Ipsen (Inst), IQVIA (Inst), Medtronic (Inst), Merck Serono (Inst), MSD Oncology (Inst), Mylan (Inst), and Pfizer (Inst); and funding for travel/accommodations/expenses from AstraZeneca, Merck Serono, Merck Sharp & Dohme, Sanofi, and Deciphera Pharmaceuticals, LLC. W Reichmann reports employment with Deciphera Pharmaceuticals, LLC, and stock and other ownership interests in Deciphera Pharmaceuticals, LLC. K Sprott reports employment with Deciphera Pharmaceuticals, LLC (self), and Stablix (immediate family member); and stock and other ownership interests in Deciphera Pharmaceuticals, LLC (self), and Stablix (immediate family member). P Cox reports employment with Deciphera Pharmaceuticals, LLC (self), and stock and other ownership interests in Deciphera Pharmaceuticals, LLC. ML Sherman reports employment with Deciphera Pharmaceuticals, LLC; an independent board of directors position with Pieris Pharmaceuticals; leadership roles with Deciphera Pharmaceuticals, LLC, and Pieris Pharmaceuticals; stock/other ownership interests in Deciphera Pharmaceuticals, LLC, and Pieris Pharmaceuticals; and patents/royalties/other intellectual property from Acceleron Pharma. R Ruiz-Soto reports employment with Deciphera Pharmaceuticals, LLC; stock/other ownership interests in ImmunoGen and Deciphera Pharmaceuticals, LLC; and patents/royalties/other intellectual property from Deciphera Pharmaceuticals, LLC (inventor in pending patents at Deciphera Pharmaceuticals, LLC, transferred the rights to Deciphera Pharmaceuticals, LLC, has not received [and will not receive] any royalties), and ImmunoGen (inventor in 3 patents with ImmunoGen, transferred the rights to ImmunoGen, has not received [and will not receive] any royalties). MC Heinrich reports honorarium from Novartis; consulting/advisory roles for Blueprint Medicines, Novartis, Theseus Pharmaceuticals, and Deciphera Pharmaceuticals, LLC; patents/royalties/other intellectual property licensed to Novartis (institutional; treatment of GIST); and partial salary from a research grant from the Jonathan David Foundation, a VA Merit Review grant (I01BX005358), and an NCI R21 grant (R21CA263400). S Bauer reports honoraria from Bayer, GlaxoSmithKline, Novartis, Pfizer, PharmaMar, and Deciphera Pharmaceuticals, LLC; consulting/advisory roles with ADC Therapeutics, Adcendo, Bayer, Blueprint Medicines, Boehringer Ingelheim, Daiichi Sankyo, Exelixis, GlaxoSmithKline, Janssen-Cilag, Lilly, Mundipharma, Nanobiotix, and Deciphera Pharmaceuticals, LLC; research funding from Blueprint Medicines, Incyte (Inst), and Novartis; and funding for travel/accommodations/expenses from PharmaMar. The authors have no other competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures


References
-
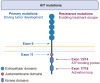
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Gastrointestinal Stromal Tumors V. 1.2024©. National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed [March 10, 2024]. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or aplication and disclaims any responsibility for their application or use in any way.
-
• Discusses clinical practice guidelines for gastrointestinal stromal tumors (GIST) developed by the National Comprehensive Cancer Network®.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
