White matter hyperintensity on MRI and plasma Aβ42/40 ratio additively increase the risk of cognitive impairment in hypertensive adults
- PMID: 39229896
- PMCID: PMC11485393
- DOI: 10.1002/alz.14126
White matter hyperintensity on MRI and plasma Aβ42/40 ratio additively increase the risk of cognitive impairment in hypertensive adults
Abstract
Introduction: Dementia often involves comorbid Alzheimer's and vascular pathology, but their combined impact warrants additional study.
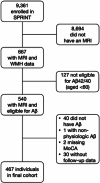
Methods: We analyzed the Systolic Blood Pressure Intervention Trial and categorized white matter hyperintensity (WMH) volume into highest versus lowest/mid tertile and the amyloid beta (Aβ)42/40 ratio into lowest versus mid/highest ratio tertile. Using these binary variables, we created four exposure categories: (1) combined low risk, (2) Aβ risk, (3) WMH risk, and (4) combined high risk.
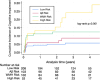
Results: In the cohort of 467 participants (mean age 69.7 ± 7.1, 41.8% female, 31.9% nonwhite or Hispanic) during 4.8 years of follow-up and across the four exposure categories the rates of cognitive impairment were 5.3%, 7.8%, 11.8%, and 22.6%. Compared to the combined low-risk category, the adjusted hazard ratio for cognitive impairment was 4.12 (95% confidence interval, 1.71 to 9.94) in the combined high-risk category.
Discussion: This study emphasizes the potential impact of therapeutic approaches to dementia prevention that target both vascular and amyloid pathology.
Highlights: White matter hyperintensity (WMH) and plasma amyloid (Aβ42/40) are additive risk factors for the development of cognitive impairment in the SPRINT MIND trial. Individuals in the high-risk categories of both WMH and Aβ42/40 had a near fivefold increase in risk of cognitive impairment during 4.8 years of follow-up on average. These findings suggest that treatment strategies targeting both vascular health and amyloid burden warrant further research.
Keywords: Alzheimer's disease biomarkers; Aβ42/40 ratio; cerebrovascular pathology; cognitive impairment risk; dementia; vascular and amyloid pathways; white matter hyperintensity (WMH).
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Dr. de Havenon has received consultant fees from Novo Nordisk, royalty fees from UpToDate, and has equity in TitinKM and Certus. Dr. Sheth reports compensation from Sense and Zoll, for data and safety monitoring services; compensation from CereVasc, CSL Behring, Rhaeos, and Astrocyte for consultant services; a patent for Stroke wearables licensed to Alva Health. Dr. Brickman serves as a scientific advisor/consultant to Cognito Therapeutics, Cognition Therapeutics, and Cogstate. He serves on data safety and monitoring boards for Albert Einstein College of Medicine and University of Illinois. Dr. Brickman has a patent for white matter hyperintensity quantification (patent # 9867566) and a patent pending for microbleed detection (publication #20230298170). Dr. Sharma has a provisional patent for an ischemic stroke etiology classification algorithm (No.63/505,006). Dr. Schneider reports serving as an Associate Editor for the journal
Figures


References
-
- Lao PJ, Chesebro AG, Beato JM, et al. Relative contribution of white matter hyperintensity to amyloid and neurodegeneration in cognitive decline over time or clinical diagnoses in a diverse, community‐based cohort of older adults. Alzheimers Dement. 2020;16:e045303.
MeSH terms
Substances
Grants and funding
- R01NR018335/NIH/NINDS
- U01NS106513/NIH/NINDS
- 1R38HL167282-01/Utah Stimulating Access to Research in Residency
- K23NS123340/American Heart Association
- U01 NS106513/NS/NINDS NIH HHS/United States
- R38 HL167282/HL/NHLBI NIH HHS/United States
- K23NS121634/Department of Defense
- R01 MD016178/MD/NIMHD NIH HHS/United States
- R01NS110721/NIH/NINDS
- R01 NR018335/NR/NINR NIH HHS/United States
- K23 NS123340/NS/NINDS NIH HHS/United States
- UG3 NS130228/NS/NINDS NIH HHS/United States
- U24 NS107136/NS/NINDS NIH HHS/United States
- U24NS107136/NIH/NINDS
- UL1 TR001863/TR/NCATS NIH HHS/United States
- U24AG082930/AG/NIA NIH HHS/United States
- R01AG055606/AG/NIA NIH HHS/United States
- U24 AG082930/AG/NIA NIH HHS/United States
- R01NS130189/NIH/NINDS
- R01 NS130189/NS/NINDS NIH HHS/United States
- UG3NS130228/NIH/NINDS
- HT9425-23-1-0981/Department of Defense
- U24NS107215/NIH/NINDS
- U24 NS107215/NS/NINDS NIH HHS/United States
- W81XWH-21-1-0590/Department of Defense
- U24NS129500/NIH/NINDS
- R01 AG065805/AG/NIA NIH HHS/United States
- R01 NS110721/NS/NINDS NIH HHS/United States
- R01MD016178/NIH/NINDS
- U24 NS129500/NS/NINDS NIH HHS/United States
- K23NS105924/NIH/NINDS
- K23 NS121634/NS/NINDS NIH HHS/United States
- R01AG065805/AG/NIA NIH HHS/United States
- K23 NS105924/NS/NINDS NIH HHS/United States
- R01EB301114/NIH/NINDS
- R01 AG055606/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

