The efficacy and functional consequences of interactions between human spermatozoa and seminal fluid extracellular vesicles
- PMID: 39230058
- PMCID: PMC11466262
- DOI: 10.1530/RAF-23-0088
The efficacy and functional consequences of interactions between human spermatozoa and seminal fluid extracellular vesicles
Abstract
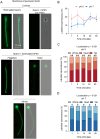
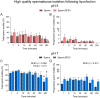
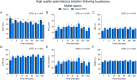
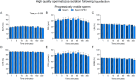
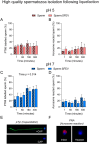
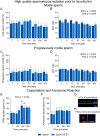
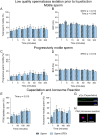
Abstract: Seminal fluid extracellular vesicles (SFEVs) have previously been shown to interact with spermatozoa and influence their fertilisation capacity. Here, we sought to extend these studies by exploring the functional consequences of SFEV interactions with human spermatozoa. SFEVs were isolated from the seminal fluid of normozoospermic donors prior to assessing the kinetics of sperm-SFEV binding in vitro, as well as the effects of these interactions on sperm capacitation, acrosomal exocytosis, and motility profile. Biotin-labelled SFEV proteins were transferred primarily to the flagellum of spermatozoa within minutes of co-incubation, although additional foci of SFEV biotinylated proteins also labelled the mid-piece and head domain. Functional analyses of high-quality spermatozoa collected following liquefaction revealed that SFEVs did not influence sperm motility during incubation at pH 5, yet SFEVs induced subtle increases in total and progressive motility in sperm incubated with SFEVs at pH 7. Additional investigation of sperm motility kinematic parameters revealed that SFEVs significantly decreased beat cross frequency and increased distance straight line, linearity, straightness, straight line velocity, and wobble. SFEVs did not influence sperm capacitation status or the ability of sperm to undergo acrosomal exocytosis. Functional assessment of both high- and low-quality spermatozoa collected prior to liquefaction showed limited SFEV influence, with these vesicles inducing only subtle decreases in beat cross frequency in spermatozoa of both groups. These findings raise the prospect that, aside from subtle effects on sperm motility, the encapsulated SFEV cargo may be destined for physiological targets other than the male germline, notably the female reproductive tract.
Lay summary: A male's influence over the biological processes of pregnancy extends beyond the provision of sperm. Molecular signals present in the ejaculate can influence the likelihood of pregnancy and healthy pregnancy progression, but the identity and function of these signals remain unclear. In this study, we wanted to understand if nano-sized particles present in the male ejaculate, called seminal fluid extracellular vesicles, can assist sperm in traversing the female reproductive tract to access the egg. To explore this, we isolated seminal fluid extracellular vesicles from human semen and incubated them with sperm. Our data showed that seminal fluid extracellular vesicles act to transfer molecular information to sperm, but this resulted in only subtle changes to the movement of sperm.
Keywords: extracellular vesicles; fertility; seminal fluid; sperm capacitation; sperm motility; spermatozoa.
Conflict of interest statement
BN is a member of the Editorial Board of
Figures


References
-
- Aalberts M Van Dissel-Emiliani FMF Van Adrichem NPH Van Wijnen M Wauben MHM Stout TAE & Stoorvogel W. 2012Identification of distinct populations of prostasomes that differentially express prostate stem cell antigen, annexin A1, and GLIPR2 in humans. Biology of Reproduction 8682. (10.1095/biolreprod.111.095760) - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

