Europium(II/III) coordination chemistry toward applications
- PMID: 39230388
- PMCID: PMC11373536
- DOI: 10.1039/d4cc03080j
Europium(II/III) coordination chemistry toward applications
Abstract
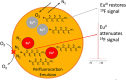
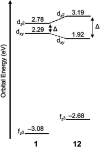
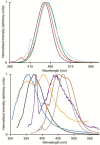
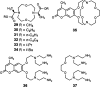
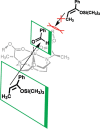
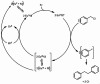
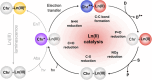
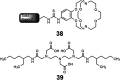
Europium is an f-block metal with two easily accessible oxidation states (+2 and +3) that have vastly different magnetic and optical properties from each other. These properties are tunable using coordination chemistry and are useful in a variety of applications, including magnetic resonance imaging, luminescence, and catalysis. This review describes important aspects of coordination chemistry of Eu from the Allen Research Group and others, how ligand design has tuned the properties of Eu ions, and how those properties are relevant to specific applications. The review begins with an introduction to the coordination chemistry of divalent and trivalent Eu followed by examples of how the coordination chemistry of Eu has made contributions to magnetic resonance imaging, luminescence, catalysis, and separations. The article concludes with a brief outlook on future opportunities in the field.
Conflict of interest statement
There are no conflicts to declare.
Figures



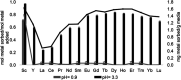





References
-
- Carnall W. T. Fields P. R. Rajnak K. J. Chem. Phys. 1968;49:4450. doi: 10.1063/1.1669896. - DOI
-
- Atomic Energy Levels–The Rare Earth Elements: The Spectra of Lanthanum, Cerium, Praseodymium, Neodynium, Promethium, Samarium, Europium, Gadolinium, Terbium, Dysprosium, Holmium, Erbium, Thulium, Ytterbium, and Lutetium, ed. W. C. Martin, R. Zalubus and L. Hagan, National Bureau of Standards, Washington D.C., 1978, p. 205
-
- Binnemans K. Coord. Chem. Rev. 2015;295:1. doi: 10.1016/j.ccr.2015.02.015. - DOI
-
- Kovacs D. Borbas E. Coord. Chem. Rev. 2018;364:1. doi: 10.1016/j.ccr.2018.03.004. - DOI
-
- Sabbatini N. Ciano M. Dellonte S. Bonazzi A. Bolletta F. Balzani V. J. Phys. Chem. 1984;88:1534. doi: 10.1021/j150652a018. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

