Re-examination of the Claimed Isolation of Stable Noncyclic 1,2-Disulfoxides
- PMID: 39230394
- PMCID: PMC11574841
- DOI: 10.1021/acs.orglett.4c02849
Re-examination of the Claimed Isolation of Stable Noncyclic 1,2-Disulfoxides
Abstract
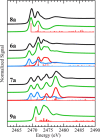
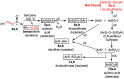
Re-examination of the claimed isolation and X-ray characterization of di-p-tolyl and dimesityl 1,2-disulfoxides from thermolysis of the corresponding aryl sulfinimines and thiosulfinates showed that the isolated disulfide dioxides are instead the well-known isomeric thiosulfonates, as confirmed by XAS, DART-MS, X-ray, IR and NMR methods. Concerns with the original X-ray structures are addressed. Our results agree with the DFT prediction of very weak diaryl 1,2-disulfoxide S-S bond dissociation enthalpies. For now, room-temperature-stable noncyclic 1,2-disulfoxides remain unknown.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
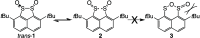
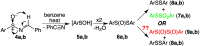
References
-
- Block S. S.; Weidner J. P. Vibrational Behavior and Structure of Disulfide Dioxides (Thiolsulfonates). Appl. Spectrosc. 1966, 20 (2), 73–79. 10.1366/000370266774386272. - DOI
-
- Cymerman J.; Willis J. B. The Infra-red Spectra and Chemical Structure of Some Aromatic Disulphides, Disulphones, and Thiolsulphonates. J. Chem. Soc. 1951, 1332–1337. 10.1039/jr9510001332. - DOI
-
- Sweetman B. J. Structure of “Cystine Disulfoxide”. Nature 1959, 183, 744–745. 10.1038/183744b0. - DOI
-
- Chau M. M.; Kice J. L. A Search for an α-Disulfoxide as an Intermediate in the Oxidation of an Aryl Thiolsulfinate. J. Am. Chem. Soc. 1976, 98 (24), 7711–7716. 10.1021/ja00440a043. - DOI
-
- Block E.; Bayer T. (Z,Z)-d,l-2,3-Dimethyl-1,4-butanedithial 1,4-Dioxide: A Novel Biologically Active Organosulfur Compound from Onion. Formation of vic-Disulfoxides in Onion Extracts. J. Am. Chem. Soc. 1990, 112 (11), 4584–4585. 10.1021/ja00167a089. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

