Aficamten and Cardiopulmonary Exercise Test Performance: A Substudy of the SEQUOIA-HCM Randomized Clinical Trial
- PMID: 39230885
- PMCID: PMC11375526
- DOI: 10.1001/jamacardio.2024.2781
Aficamten and Cardiopulmonary Exercise Test Performance: A Substudy of the SEQUOIA-HCM Randomized Clinical Trial
Abstract
Importance: Impaired exercise capacity is a cardinal manifestation of obstructive hypertrophic cardiomyopathy (HCM). The Phase 3 Trial to Evaluate the Efficacy and Safety of Aficamten Compared to Placebo in Adults With Symptomatic Obstructive HCM (SEQUOIA-HCM) is a pivotal study characterizing the treatment effect of aficamten, a next-in-class cardiac myosin inhibitor, on a comprehensive set of exercise performance and clinical measures.
Objective: To evaluate the effect of aficamten on exercise performance using cardiopulmonary exercise testing with a novel integrated measure of maximal and submaximal exercise performance and evaluate other exercise measures and clinical correlates.
Design, setting, and participants: This was a prespecified analysis from SEQUOIA-HCM, a double-blind, placebo-controlled, randomized clinical trial. Patients were recruited from 101 sites in 14 countries (North America, Europe, Israel, and China). Individuals with symptomatic obstructive HCM with objective exertional intolerance (peak oxygen uptake [pVO2] ≤90% predicted) were included in the analysis. Data were analyzed from January to March 2024.
Interventions: Randomized 1:1 to aficamten (5-20 mg daily) or matching placebo for 24 weeks.
Main outcomes and measures: The primary outcome was change from baseline to week 24 in integrated exercise performance, defined as the 2-component z score of pVO2 and ventilatory efficiency throughout exercise (minute ventilation [VE]/carbon dioxide output [VCO2] slope). Response rates for achieving clinically meaningful thresholds for change in pVO2 and correlations with clinical measures of treatment effect (health status, echocardiographic/cardiac biomarkers) were also assessed.
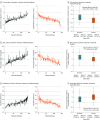
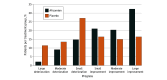
Results: Among 282 randomized patients (mean [SD] age, 59.1 [12.9] years; 115 female [40.8%], 167 male [59.2%]), 263 (93.3%) had core laboratory-validated exercise testing at baseline and week 24. Integrated composite exercise performance improved in the aficamten group (mean [SD] z score, 0.17 [0.51]) from baseline to week 24, whereas the placebo group deteriorated (mean [SD] z score, -0.19 [0.45]), yielding a placebo-corrected improvement of 0.35 (95% CI, 0.25-0.46; P <.001). Further, aficamten treatment demonstrated significant improvements in total workload, circulatory power, exercise duration, heart rate reserve, peak heart rate, ventilatory efficiency, ventilatory power, and anaerobic threshold (all P <.001). In the aficamten group, large improvements (≥3.0 mL/kg per minute) in pVO2 were more common than large reductions (32% and 2%, respectively) compared with placebo (16% and 11%, respectively). Improvements in both components of the primary outcome, pVO2 and VE/VCO2 slope throughout exercise, were significantly correlated with improvements in symptom burden and hemodynamics (all P <.05).
Conclusions and relevance: This prespecified analysis of the SEQUOIA-HCM randomized clinical trial found that aficamten treatment improved a broad range of exercise performance measures. These findings offer valuable insight into the therapeutic effects of aficamten.
Trial registration: ClinicalTrials.gov Identifier: NCT05186818.
Conflict of interest statement
Figures

References
-
- Ommen SR, Mital S, Burke MA, et al. . 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy—executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2020;142(25):e533-e557. doi:10.1161/CIR.0000000000000938 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

