Outcomes of Adjunctive Corticosteroid Treatment in Hypoxemic Adults Hospitalized for Mycoplasma pneumoniae Pneumonia: A Retrospective Cohort Study
- PMID: 39230949
- PMCID: PMC11848262
- DOI: 10.1093/cid/ciae451
Outcomes of Adjunctive Corticosteroid Treatment in Hypoxemic Adults Hospitalized for Mycoplasma pneumoniae Pneumonia: A Retrospective Cohort Study
Abstract
Background: Corticosteroids appears to be beneficial for severe Mycoplasma pneumoniae pneumonia in children, but data in adults are limited. This study investigated effects of adjunctive corticosteroids in hypoxemic adults with M. pneumoniae pneumonia.
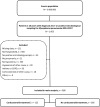
Methods: Adults admitted 2013-2017 with verified M. pneumoniae pneumonia and hypoxemia (SpO2 < 93% or oxygen treatment) were included in a cohort. Treatment was defined as receipt of at least 1 glucocorticoid dose.Primary outcome was time to regression of hypoxemia, analyzed with a multivariable Cox regression. Secondary outcomes included fever duration, length of stay, and complications.
Results: Corticosteroids were given to 31% (122/388) during hypoxemia. Median age was 44 (interquartile range [IQR] 34-57) years. Median time to start of corticosteroid treatment was 1.9 (IQR 0.6-3.6) days from admission. Median cumulative dose was equivalent to 15 (IQR 10-19) mg betamethasone. Treatment duration was 5 (IQR 3-6) days. Patients treated with corticosteroids had more severe respiratory disease, longer symptom duration, and were more often treated with fluoroquinolones.Time to regression of hypoxemia (hazard ratio [HR] 0.92 [95% confidence interval {CI}: .72-1.19], P = .53) and length of stay (HR 0.91 [95% CI: .71-1.16], P = .44) were not significantly different between corticosteroid treated and controls. Corticosteroid treatment was associated to shorter fever duration (HR 1.44 [95% CI: 1.00-2.06], P = .046). Complications did not differ significantly between treatment groups.
Conclusions: Adjunctive corticosteroids were not associated with reduced time to regression of hypoxemia in adults with M. pneumoniae pneumonia. However, duration of fever was shorter and no increase in complications was seen.
Keywords: Mycoplasma pneumoniae; adults; corticosteroids; pneumonia; treatment.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. A. N. reports receiving payments for lectures from Mediahuset, for expert testimony from IQVIA and Securis, and for participating in Data Safety Monitoring Boards (DSMB) or Advisory Boards from Astra Zeneca, Pfizer, and Jansen Pharmaceuticals. All outside of the submitted work. J. U. reports participating in DSMBs for 2 academic malaria studies, outside of the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- Foy HM. Infections caused by Mycoplasma pneumoniae and possible carrier state in different populations of patients. Clin Infect Dis 1993; 17(Suppl 1):S37–46. - PubMed
-
- Clyde WA Jr. Clinical overview of typical Mycoplasma pneumoniae infections. Clin Infect Dis 1993; 17(Suppl 1):S32–6. - PubMed
-
- Qiu JL, Huang L, Shao MY, et al. Efficacy and safety of azithromycin combined with glucocorticoid on refractory Mycoplasma pneumoniae pneumonia in children: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2020; 99:e20121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical