Cerebrospinal fluid flow extends to peripheral nerves further unifying the nervous system
- PMID: 39231237
- PMCID: PMC11373606
- DOI: 10.1126/sciadv.adn3259
Cerebrospinal fluid flow extends to peripheral nerves further unifying the nervous system
Abstract
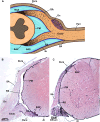
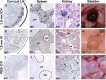
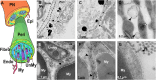
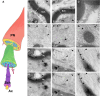
Cerebrospinal fluid (CSF) is responsible for maintaining brain homeostasis through nutrient delivery and waste removal for the central nervous system (CNS). Here, we demonstrate extensive CSF flow throughout the peripheral nervous system (PNS) by tracing distribution of multimodal 1.9-nanometer gold nanoparticles, roughly the size of CSF circulating proteins, infused within the lateral cerebral ventricle (a primary site of CSF production). CSF-infused 1.9-nanometer gold transitions from CNS to PNS at root attachment/transition zones and distributes through the perineurium and endoneurium, with ultimate delivery to axoplasm of distal peripheral nerves. Larger 15-nanometer gold fails to transit from CNS to PNS and instead forms "dye-cuffs," as predicted by current dogma of CSF restriction within CNS, identifying size limitations in central to peripheral flow. Intravenous 1.9-nanometer gold is unable to cross the blood-brain/nerve barrier. Our findings suggest that CSF plays a consistent role in maintaining homeostasis throughout the nervous system with implications for CNS and PNS therapy and neural drug delivery.
Figures
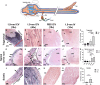
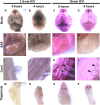

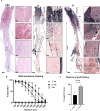
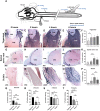

Update of
-
Cerebrospinal Fluid Flow Extends to Peripheral Nerves.bioRxiv [Preprint]. 2023 Nov 21:2023.11.20.567884. doi: 10.1101/2023.11.20.567884. bioRxiv. 2023. Update in: Sci Adv. 2024 Sep 6;10(36):eadn3259. doi: 10.1126/sciadv.adn3259. PMID: 38045235 Free PMC article. Updated. Preprint.
References
-
- Sakka L., Coll G., Chazal J., Anatomy and physiology of cerebrospinal fluid. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 128, 309–316 (2011). - PubMed
-
- Iliff J., Wang M., Liao Y., Plogg B., Peng W., Gundersen G., Benveniste H., Vates G., Deane R., Goldman S., Nagelhus E., Nedergaard M., A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 4, 147ra111 (2012). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

