Adjunctive Intravenous Argatroban or Eptifibatide for Ischemic Stroke
- PMID: 39231343
- PMCID: PMC11528349
- DOI: 10.1056/NEJMoa2314779
Adjunctive Intravenous Argatroban or Eptifibatide for Ischemic Stroke
Abstract
Background: Intravenous thrombolysis is a standard treatment of acute ischemic stroke. The efficacy and safety of combining intravenous thrombolysis with argatroban (an anticoagulant agent) or eptifibatide (an antiplatelet agent) are unclear.
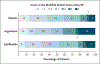
Methods: We conducted a phase 3, three-group, adaptive, single-blind, randomized, controlled clinical trial at 57 sites in the United States. Patients with acute ischemic stroke who had received intravenous thrombolysis within 3 hours after symptom onset were assigned to receive intravenous argatroban, eptifibatide, or placebo within 75 minutes after the initiation of thrombolysis. The primary efficacy outcome, the utility-weighted 90-day modified Rankin scale score (range, 0 to 10, with higher scores reflecting better outcomes), was assessed by means of centralized adjudication. The primary safety outcome was symptomatic intracranial hemorrhage within 36 hours after randomization.
Results: A total of 514 patients were assigned to receive argatroban (59 patients), eptifibatide (227 patients), or placebo (228 patients). All the patients received intravenous thrombolysis (70% received alteplase, and 30% received tenecteplase), and 225 patients (44%) underwent endovascular thrombectomy. At 90 days, the mean (±SD) utility-weighted modified Rankin scale scores were 5.2±3.7 with argatroban, 6.3±3.2 with eptifibatide, and 6.8±3.0 with placebo. The posterior probability that argatroban was better than placebo was 0.002 (posterior mean difference in utility-weighted modified Rankin scale score, -1.51±0.51) and that eptifibatide was better than placebo was 0.041 (posterior mean difference, -0.50±0.29). The incidence of symptomatic intracranial hemorrhage was similar in the three groups (4% with argatroban, 3% with eptifibatide, and 2% with placebo). Mortality at 90 days was higher in the argatroban group (24%) and the eptifibatide group (12%) than in the placebo group (8%).
Conclusions: In patients with acute ischemic stroke treated with intravenous thrombolysis within 3 hours after symptom onset, adjunctive treatment with intravenous argatroban or eptifibatide did not reduce poststroke disability and was associated with increased mortality. (Funded by the National Institute of Neurological Disorders and Stroke; MOST ClinicalTrials.gov number, NCT03735979.).
Copyright © 2024 Massachusetts Medical Society.
Figures

References
-
- Alexandrov AV, Grotta JC. Arterial re-occlusion in stroke patients treated with intravenous tissue plasminogen activator. Neurology 2002;59:862–7. - PubMed
-
- Muchada M, Rodriguez-Luna D, Pagola J, et al. Impact of time to treatment on tissue-type plasminogen activator-induced recanalization in acute ischemic stroke. Stroke 2014;45:2734–8. - PubMed
-
- Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–31. - PubMed
-
- Jeske WP, Fareed J, Hoppensteadt DA, Lewis B, Walenga JM. Pharmacology of argatroban. Expert Rev Hematol 2010;3:527–39. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
