Lysosome-Targeted Bifunctional Therapeutics Induce Autodynamic Cancer Therapy
- PMID: 39231370
- PMCID: PMC11538690
- DOI: 10.1002/advs.202401424
Lysosome-Targeted Bifunctional Therapeutics Induce Autodynamic Cancer Therapy
Abstract
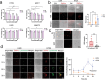
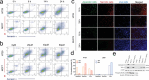
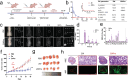
Autodynamic cancer therapy possesses tremendous potential for enhancing therapeutic efficacy by initiating the treatment process autonomously within targeted cells. However, challenges related to biocompatibility and targeted delivery have hindered its clinical translation owing to the induction of adverse effects and cytotoxicity in healthy cells. In this study, a novel approach for auto-initiated dynamic therapy by conjugating zwitterionic near-infrared fluorophores to a cell-penetrating peptide is proposed. This enables efficient cellular uptake and specific targeting of therapy to desired cells while avoiding off-target uptake. The zwitterionic bioconjugate causes cancer-specific toxicity following its internalization into the targeted cells, triggered by specific intracellular conditions in lysosomes. This innovative approach enables selective targeting of lysosomes in malignant cells while minimizing cytotoxic effects on normal cells. By targeting lysosomes, the method overcomes inherent risks and side effects associated with conventional cancer treatments, offering a selective and effective approach to cancer therapy.
Keywords: anticancer agent; cell‐penetrating peptide; fluorescence dye; near‐infrared imaging; targeting therapy.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
