Personalized Therapy Selection by Integration of Molecular Cancer Classification by the 92-Gene Assay and Tumor Profiling in Patients With Cancer of Unknown Primary
- PMID: 39231374
- PMCID: PMC11382827
- DOI: 10.1200/PO.24.00191
Personalized Therapy Selection by Integration of Molecular Cancer Classification by the 92-Gene Assay and Tumor Profiling in Patients With Cancer of Unknown Primary
Abstract
Purpose: Cancer of unknown primary (CUP) is a syndrome comprising metastatic cancers without a clinically identified primary site. Although patients with CUP have an unfavorable prognosis, treatment with site-specific therapies guided by clinical features, standard pathology, and molecular assays can improve overall survival. The 92-gene assay (CancerTYPE ID) is a gene expression-based classifier that helps identify the tissue of origin for metastatic cancers with unknown or uncertain diagnoses. This study reports the frequency of selected molecular aberrations of oncogenes, including KRAS, IDH1/2, BRCA1/2, and BRAF, in patients with CUP in the MOSAIC database to highlight potential treatment options.
Methods: MOSAIC is a database of patients with CUP submitted for CancerTYPE ID testing and NeoTYPE biomarker testing. Tumor biopsy samples were analyzed by CancerTYPE ID for tumor type identification and further tested for molecular aberrations of oncogenes, including KRAS, IDH1/2, BRCA1/2, and BRAF.
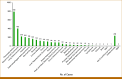
Results: CancerTYPE ID identified a specific tumor type in 92.5% (2,929 of 3,168) of CUP cases in the MOSAIC database. The most commonly identified histological type was adenocarcinoma (75.4%), with pancreaticobiliary being the most common molecularly diagnosed cancer (24.9%). Aberrations in KRAS, IDH1/2, BRCA, and BRAF genes were identified in 18.8% (n = 597) of biopsies. A cancer-specific US Food and Drug Administration (FDA)-approved or investigational targeted therapy was potentially available for 24.6% (n = 147) of these patients.
Conclusion: This retrospective analysis supports incorporating CancerTYPE ID into the evaluation for patients with CUP to help determine the tissue of origin and identify actionable genetic alterations. This approach may allow more patients with CUP to benefit from site-specific FDA-approved targeted therapies or enrollment into clinical trials.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures

References
-
- Olivier T, Fernandez E, Labidi-Galy I, et al. : Redefining cancer of unknown primary: Is precision medicine really shifting the paradigm? Cancer Treat Rev 97:102204, 2021 - PubMed
-
- Ettinger DS, Stevenson MM, Mutar SA, et al. : Occult primary (cancer of unknown primary [CUP]). https://www.nccn.org/professionals/physician_gls/pdf/occult.pdf
-
- Varadhachary GR, Greco FA: Overview of patient management and future directions in unknown primary carcinoma. Semin Oncol 36:75-80, 2009 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

