Electroanalytical Strategies for Local pH Sensing at Solid-Liquid Interfaces and Biointerfaces
- PMID: 39231377
- PMCID: PMC11443533
- DOI: 10.1021/acssensors.4c01391
Electroanalytical Strategies for Local pH Sensing at Solid-Liquid Interfaces and Biointerfaces
Abstract
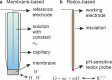
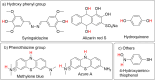
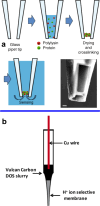
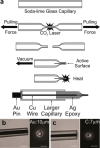
Obtaining analytical information about chemical species at interfaces is fundamentally important to improving our understanding of chemical reactions and biological processes. pH at solid-liquid interfaces is found to deviate from the bulk solution value, for example, in electrocatalytic reactions at surfaces or during the corrosion of metals. Also, in the vicinity of living cells, metabolic reactions or cellular responses cause changes in pH at the extracellular interface. In this review, we collect recent progress in the development of sensors with the capability to detect pH at or close to solid-liquid and bio interfaces, with spatial and time resolution. After the two main principles of pH detection are presented, the different classes of molecules and materials that are used as active components in these sensors are described. The review then focuses on the reported electroanalytical techniques for local pH sensing. As application examples, we discuss model studies that exploit local pH sensing in the area of electrocatalysis, corrosion, and cellular interfaces. We conclude with a discussion of key challenges for wider use of this analytical approach, which shows promise to improve the mechanistic understanding of reactions and processes at realistic interfaces.
Keywords: corrosion; electrified interface; electrocatalysis; extracellular pH; ion selective electrode; pH sensor; potentiometry; scanning electrochemical microscopy; ultramicroelectrode; voltammetry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




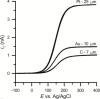

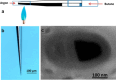


References
-
- Sørensen S. P. L. Über die Messung und die Bedeutung der Wasserstoffionenkonzentration bei enzymatischen Prozessen. Biochemische Zeitschrift 1909, (21), 131–200.
-
- Figueiredo M. C.; Arán-Ais R. M.; Climent V.; Kallio T.; Feliu J. M. Evidence of Local pH Changes during Ethanol Oxidation at Pt Electrodes in Alkaline Media. ChemElectroChem 2015, 2 (9), 1254–1258. 10.1002/celc.201500151. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

