Early intermittent hyperlipidaemia alters tissue macrophages to fuel atherosclerosis
- PMID: 39231480
- PMCID: PMC11464399
- DOI: 10.1038/s41586-024-07993-x
Early intermittent hyperlipidaemia alters tissue macrophages to fuel atherosclerosis
Abstract
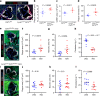
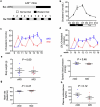
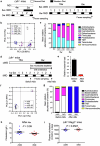
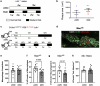
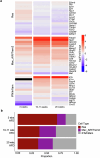
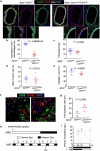
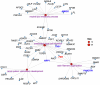
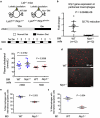
Hyperlipidaemia is a major risk factor of atherosclerotic cardiovascular disease (ASCVD). Risk of cardiovascular events depends on cumulative lifetime exposure to low-density lipoprotein cholesterol (LDL-C) and, independently, on the time course of exposure to LDL-C, with early exposure being associated with a higher risk1. Furthermore, LDL-C fluctuations are associated with ASCVD outcomes2-4. However, the precise mechanisms behind this increased ASCVD risk are not understood. Here we find that early intermittent feeding of mice on a high-cholesterol Western-type diet (WD) accelerates atherosclerosis compared with late continuous exposure to the WD, despite similar cumulative circulating LDL-C levels. We find that early intermittent hyperlipidaemia alters the number and homeostatic phenotype of resident-like arterial macrophages. Macrophage genes with altered expression are enriched for genes linked to human ASCVD in genome-wide association studies. We show that LYVE1+ resident macrophages are atheroprotective, and identify biological pathways related to actin filament organization, of which alteration accelerates atherosclerosis. Using the Young Finns Study, we show that exposure to cholesterol early in life is significantly associated with the incidence and size of carotid atherosclerotic plaques in mid-adulthood. In summary, our results identify early intermittent exposure to cholesterol as a strong determinant of accelerated atherosclerosis, highlighting the importance of optimal control of hyperlipidaemia early in life, and providing insights into the underlying biological mechanisms. This knowledge will be essential to designing effective therapeutic strategies to combat ASCVD.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Comment in
-
Lower your cholesterol early, and stick with it!Nat Rev Cardiol. 2025 Feb;22(2):69-70. doi: 10.1038/s41569-024-01095-x. Nat Rev Cardiol. 2025. PMID: 39424909 No abstract available.
References
-
- Domanski, M. J. et al. Time course of LDL cholesterol exposure and cardiovascular disease event risk. J. Am. Coll. Cardiol.76, 1507–1516 (2020). - PubMed
-
- Kim, M. K. et al. Associations of variability in blood pressure, glucose and cholesterol concentrations, and body mass index with mortality and cardiovascular outcomes in the general population. Circulation138, 2627–2637 (2018). - PubMed
-
- Bangalore, S. et al. Visit-to-visit low-density lipoprotein cholesterol variability and risk of cardiovascular outcomes: insights from the TNT trial. J. Am. Coll. Cardiol.65, 1539–1548 (2015). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

