A new mouse model for PRPH2 pattern dystrophy exhibits functional compensation prior and subsequent to retinal degeneration
- PMID: 39231530
- PMCID: PMC11540925
- DOI: 10.1093/hmg/ddae128
A new mouse model for PRPH2 pattern dystrophy exhibits functional compensation prior and subsequent to retinal degeneration
Abstract
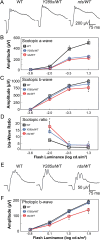
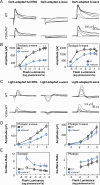
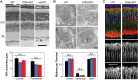
Mutations in PRPH2 are a relatively common cause of sight-robbing inherited retinal degenerations (IRDs). Peripherin-2 (PRPH2) is a photoreceptor-specific tetraspanin protein that structures the disk rim membranes of rod and cone outer segment (OS) organelles, and is required for OS morphogenesis. PRPH2 is noteworthy for its broad spectrum of disease phenotypes; both inter- and intra-familial heterogeneity have been widely observed and this variability in disease expression and penetrance confounds efforts to understand genotype-phenotype correlations and pathophysiology. Here we report the generation and initial characterization of a gene-edited animal model for PRPH2 disease associated with a nonsense mutation (c.1095:C>A, p.Y285X), which is predicted to truncate the peripherin-2 C-terminal domain. Young (P21) Prph2Y285X/WT mice developed near-normal photoreceptor numbers; however, OS membrane architecture was disrupted, OS protein levels were reduced, and in vivo and ex vivo electroretinography (ERG) analyses found that rod and cone photoreceptor function were each severely reduced. Interestingly, ERG studies also revealed that rod-mediated downstream signaling (b-waves) were functionally compensated in the young animals. This resiliency in retinal function was retained at P90, by which time substantial IRD-related photoreceptor loss had occurred. Altogether, the current studies validate a new mouse model for investigating PRPH2 disease pathophysiology, and demonstrate that rod and cone photoreceptor function and structure are each directly and substantially impaired by the Y285X mutation. They also reveal that Prph2 mutations can induce a functional compensation that resembles homeostatic plasticity, which can stabilize rod-derived signaling, and potentially dampen retinal dysfunction during some PRPH2-associated IRDs.
Keywords: PRPH2; homeostatic plasticity; inherited retinal degeneration; peripherin-2; rod and cone photoreceptors.
© The Author(s) 2024. Published by Oxford University Press.
Figures





References
-
- Boon CJ, Den Hollander AI, Hoyng CB. et al. The spectrum of retinal dystrophies caused by mutations in the peripherin/RDS gene. Prog Retin Eye Res 2008;27:213–235. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

