Targeting IL-33 reprograms the tumor microenvironment and potentiates antitumor response to anti-PD-L1 immunotherapy
- PMID: 39231544
- PMCID: PMC11409265
- DOI: 10.1136/jitc-2024-009236
Targeting IL-33 reprograms the tumor microenvironment and potentiates antitumor response to anti-PD-L1 immunotherapy
Erratum in
-
Correction: Targeting IL-33 reprograms the tumor microenvironment and potentiates antitumor response to anti-PD-L1 immunotherapy.J Immunother Cancer. 2024 Nov 21;12(11):e009236corr1. doi: 10.1136/jitc-2024-009236corr1. J Immunother Cancer. 2024. PMID: 39577871 Free PMC article. No abstract available.
Abstract
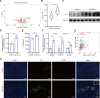
Background: The main challenge against patients with cancer to derive benefits from immune checkpoint inhibitors targeting PD-1/PD-L1 appears to be the immunosuppressive tumor microenvironment (TME), in which IL-33/ST2 signal fulfills critical functions. However, whether IL-33 limits the therapeutic efficacy of anti-PD-L1 remains uncertain.
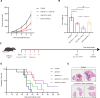
Methods: Molecular mechanisms of IL-33/ST2 signal on anti-PD-L1 treatment lewis lung carcinoma tumor model were assessed by RNA-seq, ELISA, WB and immunofluorescence (IF). A sST2-Fc fusion protein was constructed for targeting IL-33 and combined with anti-PD-L1 antibody for immunotherapy in colon and lung tumor models. On this basis, bifunctional fusion proteins were generated for PD-L1-targeted blocking of IL-33 in tumors. The underlying mechanisms of dual targeting of IL-33 and PD-L1 revealed by RNA-seq, scRNA-seq, FACS, IF and WB.
Results: After anti-PD-L1 administration, tumor-infiltrating ST2+ regulatory T cells (Tregs) were elevated. Blocking IL-33/ST2 signal with sST2-Fc fusion protein potentiated antitumor efficacy of PD-L1 antibody by enhancing T cell responses in tumor models. Bifunctional fusion protein anti-PD-L1-sST2 exhibited enhanced antitumor efficacy compared with combination therapy, not only inhibited tumor progression and extended the survival, but also provided long-term protective antitumor immunity. Mechanistically, the superior antitumor activity of targeting IL-33 and PD-L1 originated from reducing immunosuppressive factors, such as Tregs and exhausted CD8+ T cells while increasing tumor-infiltrating cytotoxic T lymphocyte cells.
Conclusions: In this study, we demonstrated that IL-33/ST2 was involved in the immunosuppression mechanism of PD-L1 antibody therapy, and blockade by sST2-Fc or anti-PD-L1-sST2 could remodel the inflammatory TME and induce potent antitumor effect, highlighting the potential therapeutic strategies for the tumor treatment by simultaneously targeting IL-33 and PD-L1.
Keywords: Immune Checkpoint Inhibitor; Immune modulatory; Immunotherapy; T regulatory cell - Treg; Tumor microenvironment - TME.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
