Genetics Navigator: protocol for a mixed methods randomized controlled trial evaluating a digital platform to deliver genomic services in Canadian pediatric and adult populations
- PMID: 39231549
- PMCID: PMC11407190
- DOI: 10.1136/bmjopen-2024-090084
Genetics Navigator: protocol for a mixed methods randomized controlled trial evaluating a digital platform to deliver genomic services in Canadian pediatric and adult populations
Abstract
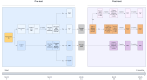
Introduction: Genetic testing is used across medical disciplines leading to unprecedented demand for genetic services. This has resulted in excessive waitlists and unsustainable pressure on the standard model of genetic healthcare. Alternative models are needed; e-health tools represent scalable and evidence-based solution. We aim to evaluate the effectiveness of the Genetics Navigator, an interactive patient-centred digital platform that supports the collection of medical and family history, provision of pregenetic and postgenetic counselling and return of genetic testing results across paediatric and adult settings.
Methods and analysis: We will evaluate the effectiveness of the Genetics Navigator combined with usual care by a genetics clinician (physician or counsellor) to usual care alone in a randomised controlled trial. One hundred and thirty participants (adults patients or parents of paediatric patients) eligible for genetic testing through standard of care will be recruited across Ontario genetics clinics. Participants randomised into the intervention arm will use the Genetics Navigator for pretest and post-test genetic counselling and results disclosure in conjunction with their clinician. Participants randomised into the control arm will receive usual care, that is, clinician-delivered pretest and post-test genetic counselling, and results disclosure. The primary outcome is participant distress 2 weeks after test results disclosure. Secondary outcomes include knowledge, decisional conflict, anxiety, empowerment, quality of life, satisfaction, acceptability, digital health literacy and health resource use. Quantitative data will be analysed using statistical hypothesis tests and regression models. A subset of participants will be interviewed to explore user experience; data will be analysed using interpretive description. A cost-effectiveness analysis will examine the incremental cost of the Navigator compared with usual care per unit reduction in distress or unit improvement in quality of life from public payer and societal perspectives.
Ethics and dissemination: This study was approved by Clinical Trials Ontario. Results will be shared through stakeholder workshops, national and international conferences and peer-reviewed journals.
Trial registration number: NCT06455384.
Keywords: delivery of health care, integrated; eHealth; genetics; health services; methods; randomized controlled trial.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: YB and MC are cofounders of Genetics Adviser.
Figures
References
-
- GeneDx. 2000. [1-Mar-2019]. https://www.genedx.com Available. Accessed.
-
- Concert Genetics Landscape of genetic testing market growth, reimbursement trends, challenges and opportunities. Am J Hum Genet. 2018
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
