Arrested development: the dysfunctional life history of medulloblastoma
- PMID: 39231614
- PMCID: PMC11789489
- DOI: 10.1101/gad.351936.124
Arrested development: the dysfunctional life history of medulloblastoma
Abstract
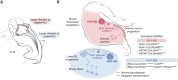
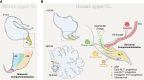
Medulloblastoma is a heterogeneous embryonal tumor of the cerebellum comprised of four distinct molecular subgroups that differ in their developmental origins, genomic landscapes, clinical presentation, and survival. Recent characterization of the human fetal cerebellum at single-cell resolution has propelled unprecedented insights into the cellular origins of medulloblastoma subgroups, including those underlying previously elusive groups 3 and 4. In this review, the molecular pathogenesis of medulloblastoma is examined through the lens of cerebellar development. In addition, we discuss how enhanced understanding of medulloblastoma origins has the potential to refine disease modeling for the advancement of treatment and outcomes.
Keywords: cerebellum; developmental origins; medulloblastoma; mouse models; organoids; single-cell genomics; stem cell models.
© 2025 Tao et al.; Published by Cold Spring Harbor Laboratory Press.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
