Microenvironmental acidification by pneumococcal sugar consumption fosters barrier disruption and immune suppression in the human alveolus
- PMID: 39231629
- PMCID: PMC11635383
- DOI: 10.1183/13993003.01983-2023
Microenvironmental acidification by pneumococcal sugar consumption fosters barrier disruption and immune suppression in the human alveolus
Abstract
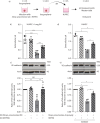
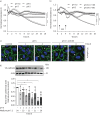
Streptococcus pneumoniae is the most common causative agent of community-acquired pneumonia worldwide. A key pathogenic mechanism that exacerbates severity of disease is the disruption of the alveolar-capillary barrier. However, the specific virulence mechanisms responsible for this in the human lung are not yet fully understood. In this study, we infected living human lung tissue with Strep. pneumoniae and observed a significant degradation of the central junctional proteins occludin and vascular endothelial cadherin, indicating barrier disruption. Surprisingly, neither pneumolysin, bacterial hydrogen peroxide nor pro-inflammatory activation were sufficient to cause this junctional degradation. Instead, pneumococcal infection led to a significant decrease of pH (∼6), resulting in the acidification of the alveolar microenvironment, which was linked to junctional degradation. Stabilising the pH at physiological levels during infection reversed this effect, even in a therapeutic-like approach. Further analysis of bacterial metabolites and RNA sequencing revealed that sugar consumption and subsequent lactate production were the major factors contributing to bacterially induced alveolar acidification, which also hindered the release of critical immune factors. Our findings highlight bacterial metabolite-induced acidification as an independent virulence mechanism for barrier disruption and inflammatory dysregulation in pneumonia. Thus, our data suggest that strictly monitoring and buffering alveolar pH during infections caused by fermentative bacteria could serve as an adjunctive therapeutic strategy for sustaining barrier integrity and immune response.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: D. Beule reports grants from DFG and BMBF; two patent applications not related to the manuscript or project; and a leadership role as AEBC2 (Core Unit Network, unpaid); outside the submitted work. S. Hammerschmidt reports support for the present manuscript from Federal Excellence Initiative of Mecklenburg Western Pomerania and European Social Fund Grant KoInfekt (ESF_14-BM-A55-0001_16). A.D. Gruber reports support for the present manuscript from German Research Council (Grant No SFB-TR84 Z01b). A.C. Hocke reports support for the present manuscript from DFG, SFB-TR84, projects B6 and Z1a, Einstein Foundation Berlin, Einstein Center 3R. Outside the submitted work, A.C. Hocke reports grants from Charité – Zeiss Development Center for Multidimensional Microscopy. All other authors have nothing to disclose.
Figures






Comment in
-
Bacterial misappropriation of host glucose in pneumococcal pneumonia.Eur Respir J. 2024 Dec 12;64(6):2401841. doi: 10.1183/13993003.01841-2024. Print 2024 Dec. Eur Respir J. 2024. PMID: 39667785 No abstract available.
References
-
- European Centre for Disease Prevention and Control . Invasive Pneumococcal Disease – Annual Epidemiological Report for 2017. www.ecdc.europa.eu/en/publications-data/invasive-pneumococcal-disease-an.... Date last updated: 14 May 2019. Date last accessed: 26 October 2023.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
