A novel in vitro tubular model to recapitulate features of distal airways: the bronchioid
- PMID: 39231631
- PMCID: PMC11627163
- DOI: 10.1183/13993003.00562-2024
A novel in vitro tubular model to recapitulate features of distal airways: the bronchioid
Abstract
Background: Airflow limitation is the hallmark of obstructive pulmonary diseases, with the distal airways representing a major site of obstruction. Although numerous in vitro models of bronchi already exist, there is currently no culture system for obstructive diseases that reproduces the architecture and function of small airways. Here, we aimed to engineer a model of distal airways to overcome the limitations of current culture systems.
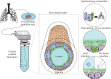
Methods: We developed a so-called bronchioid model by encapsulating human bronchial adult stem cells derived from clinical samples in a tubular scaffold made of alginate gel.
Results: This template drives the spontaneous self-organisation of epithelial cells into a tubular structure. Fine control of the level of contraction is required to establish a model of the bronchiole, which has a physiologically relevant shape and size. Three-dimensional imaging, gene expression and single-cell RNA-sequencing analysis of bronchioids made of bronchial epithelial cells revealed tubular organisation, epithelial junction formation and differentiation into ciliated and goblet cells. Ciliary beating was observed, at a decreased frequency in bronchioids made of cells from COPD patients. The bronchioid could be infected by rhinovirus. An air-liquid interface was introduced that modulated gene expression.
Conclusion: Here, we provide a proof of concept of a perfusable bronchioid with proper mucociliary and contractile functions. The key advantages of our approach, such as the air‒liquid interface, lumen accessibility, recapitulation of pathological features and possible assessment of clinically relevant end-points, will make our pulmonary organoid-like model a powerful tool for preclinical studies.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: I. Dupin and P. Berger have two patents (EP number 3050574 and EP number 20173595). I. Dupin, P. Henrot and M. Zysman report grants from the Fondation Bordeaux Université. M. Zysman reports personal fees from AstraZeneca, Boehringer Ingelheim, CSL Behring, Novartis, Chiesi and GlaxoSmithKline, and non-financial support from Lilly. P. Berger reports grants and personal fees from Novartis; personal fees and non-financial support from Chiesi, Boehringer Ingelheim, AstraZeneca and Sanofi; and personal fees from Menarini and TEVA, outside the submitted work. All other authors declare that they have no competing interests.
Figures







Comment in
-
A breath of the future: a novel human model for COPD and beyond.Eur Respir J. 2024 Dec 5;64(6):2401531. doi: 10.1183/13993003.01531-2024. Print 2024 Dec. Eur Respir J. 2024. PMID: 39638366 No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
