Angiotensin-(1-7) infusion in COVID-19 patients admitted to the ICU: a seamless phase 1-2 randomized clinical trial
- PMID: 39231898
- PMCID: PMC11374945
- DOI: 10.1186/s13613-024-01369-0
Angiotensin-(1-7) infusion in COVID-19 patients admitted to the ICU: a seamless phase 1-2 randomized clinical trial
Abstract
Background: The coronavirus-related disease (COVID-19) is mainly characterized by a respiratory involvement. The renin-angiotensin system (RAS) has a relevant role in the pathogenesis of COVID-19, as the virus enters host's cells via the angiotensin-converting enzyme 2 (ACE2).
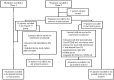
Methods: This investigator-initiated, seamless phase 1-2 randomized clinical trial was conceived to test the safety and efficacy of continuous short-term (up to 7 days) intravenous administration of Angiotensin-(1-7) in COVID-19 patients admitted to two intensive care units (ICU). In addition to standard of care, intravenous administration of Angiotensin-(1-7) was started at 5 mcg/Kg day and increased to 10 mcg/Kg day after 24 h (Phase 1; open label trial) or given at 10 mcg/Kg day and continued for a maximum of 7 days or until ICU discharge (Phase 2; double-blind randomized controlled trial). The rate of serious adverse events (SAEs) served as the primary outcome of the study for Phase 1, and the number of oxygen free days (OFDs) by day 28 for Phase 2.
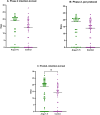
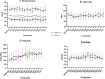
Results: Between August 2020 and July 2021, when the study was prematurely stopped due to low recruitment rate, 28 patients were included in Phase 1 and 79 patients in Phase 2. Of those, 78 were included in the intention to treat analysis, and the primary outcome was available for 77 patients. During Phase 1, one SAE (i.e., bradycardia) was considered possibly related to the infusion, justifying its discontinuation. In Phase 2, OFDs did not differ between groups (median 19 [0-21] vs. 14 [0-18] days; p = 0.15). When patients from both phases were analyzed in a pooled intention to treat approach (Phase 1-2 trial), OFDs were significantly higher in treated patients, when compared to controls (19 [0-21] vs. 14 [0-18] days; absolute difference -5 days, 95% CI [0-7] p = 0.04).
Conclusions: The main findings of our study indicate that continuous intravenous infusion of Angiotensin-(1-7) at 10 mcg/Kg day in COVID-19 patients admitted to the ICU with severe pneumonia is safe. In Phase II intention to treat analysis, there was no significant difference in OFD between groups. Trial Registration ClinicalTrials.gov Identifier: NCT04633772-Registro Brasileiro de Ensaios Clínicos, UTN number: U1111-1255-7167.
Keywords: ARDS; Angiotensin; COVID-19; Coronavirus; RAS; Renin angiotensin system.
© 2024. The Author(s).
Conflict of interest statement
The authors of the trial declared to have no conflict of interest. Prof Robson is the CEO of the company Angitec, which provided economic and logistic support without any monetary or contractual compensation.
Figures
Similar articles
-
Testing the efficacy and safety of BIO101, for the prevention of respiratory deterioration, in patients with COVID-19 pneumonia (COVA study): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Jan 11;22(1):42. doi: 10.1186/s13063-020-04998-5. Trials. 2021. PMID: 33430924 Free PMC article.
-
Management of Renin-Angiotensin-Aldosterone System blockade in patients admitted to hospital with confirmed coronavirus disease (COVID-19) infection (The McGill RAAS-COVID- 19): A structured summary of a study protocol for a randomized controlled trial.Trials. 2021 Feb 5;22(1):115. doi: 10.1186/s13063-021-05080-4. Trials. 2021. PMID: 33546734 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review.Cochrane Database Syst Rev. 2020 Oct 12;10:CD013600. doi: 10.1002/14651858.CD013600.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 May 20;5:CD013600. doi: 10.1002/14651858.CD013600.pub4. PMID: 33044747 Updated.
-
Effect of continuing the use of renin-angiotensin system inhibitors on mortality in patients hospitalized for coronavirus disease 2019: a systematic review, meta-analysis, and meta-regression analysis.BMC Infect Dis. 2023 Jan 24;23(1):53. doi: 10.1186/s12879-023-07994-7. BMC Infect Dis. 2023. PMID: 36694122 Free PMC article.
Cited by
-
Renin-angiotensin-aldosterone system activation in plasma as marker for prognosis in critically ill patients with COVID-19: a prospective exploratory study.Ann Intensive Care. 2025 Jan 16;15(1):10. doi: 10.1186/s13613-025-01433-3. Ann Intensive Care. 2025. PMID: 39821855 Free PMC article.
-
Interaction of SARS-CoV-2 and SARS-CoV-2 vaccines with renin angiotensin aldosterone system, clinical outcomes, and angiotensin (1-7) as a physiological treatment recommendation: hypothesis and theory article.Front Med (Lausanne). 2025 Jul 10;12:1612442. doi: 10.3389/fmed.2025.1612442. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40708634 Free PMC article.
-
Intranasal liposomal angiotensin-(1-7) administration reduces inflammation and viral load in the lungs during SARS-CoV-2 infection in K18-hACE2 transgenic mice.Antimicrob Agents Chemother. 2024 Dec 5;68(12):e0083524. doi: 10.1128/aac.00835-24. Epub 2024 Oct 29. Antimicrob Agents Chemother. 2024. PMID: 39470198 Free PMC article.
References
-
- https://data.who.int/dashboards/covid19/deaths?n=c- Assessed 22 Jan 2024.
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

