Targeting Fascin1 maintains chondrocytes phenotype and attenuates osteoarthritis development
- PMID: 39231936
- PMCID: PMC11374990
- DOI: 10.1038/s41413-024-00357-1
Targeting Fascin1 maintains chondrocytes phenotype and attenuates osteoarthritis development
Abstract
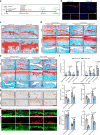
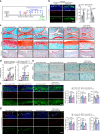
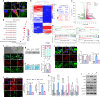
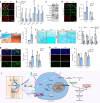
Osteoarthritis (OA) is the most common form of arthritic disease, and phenotypic modification of chondrocytes is an important mechanism that contributes to the loss of cartilage homeostasis. This study identified that Fascin actin-bundling protein 1 (FSCN1) plays a pivotal role in regulating chondrocytes phenotype and maintaining cartilage homeostasis. Proteome-wide screening revealed markedly upregulated FSCN1 protein expression in human OA cartilage. FSCN1 accumulation was confirmed in the superficial layer of OA cartilage from humans and mice, primarily in dedifferentiated-like chondrocytes, associated with enhanced actin stress fiber formation and upregulated type I and III collagens. FSCN1-inducible knockout mice exhibited delayed cartilage degeneration following experimental OA surgery. Mechanistically, FSCN1 promoted actin polymerization and disrupted the inhibition of Decorin on TGF-β1, leading to excessive TGF-β1 production and ALK1/Smad1/5 signaling activation, thus, accelerated chondrocyte dedifferentiation. Intra-articular injection of FSCN1-overexpressing adeno-associated virus exacerbated OA progression in mice, which was mitigated by an ALK1 inhibitor. Moreover, FSCN1 inhibitor NP-G2-044 effectively reduced extracellular matrix degradation in OA mice, cultured human OA chondrocytes, and cartilage explants by suppressing ALK1/Smad1/5 signaling. These findings suggest that targeting FSCN1 represents a promising therapeutic approach for OA.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

