B4GALT1-dependent galectin-8 binding with TGF-β receptor suppresses colorectal cancer progression and metastasis
- PMID: 39231945
- PMCID: PMC11375092
- DOI: 10.1038/s41419-024-07028-3
B4GALT1-dependent galectin-8 binding with TGF-β receptor suppresses colorectal cancer progression and metastasis
Abstract
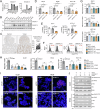
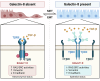
Transforming growth factor (TGF)-β signaling is critical for epithelial-mesenchymal transition (EMT) and colorectal cancer (CRC) metastasis. Disruption of Smad-depednent TGF-β signaling has been shown in CRC cells. However, TGF-β receptor remains expressed on CRC cells. Here, we investigated whether the cooperation between tumor-associated N-glycosylation and a glycan-binding protein modulated the TGF-β-driven signaling and metastasis of CRC. We showed that galectin-8, a galactose-binding lectin, hampered TGF-β-induced EMT by interacting with the type II TGF-β receptor and competing with TGF-β binding. Depletion of galectin-8 promoted the migration of CRC cells by increasing TGF-β-receptor-mediated RAS and Src signaling, which was attenuated after recombinant galectin-8 treatment. Treatment with recombinant galectin-8 also induces JNK-dependent apoptosis in CRC cells. The anti-migratory effect of galectin-8 depended on β4-galactosyltransferase-I (B4GALT1), an enzyme involved in N-glycan synthesis. Increased B4GALT1 expression was observed in clinical CRC samples. Depletion of B4GALT1 reduced the metastatic potential of CRC cells. Furthermore, inducible expression of galectin-8 attenuated tumor development and metastasis of CRC cells in an intra-splenic injection model. Our results thus demonstrate that galectin-8 alters non-canonical TGF-β response in CRC cells and suppresses CRC progression.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

