The compartment-specific manipulation of the NAD+/NADH ratio affects the metabolome and the function of glioblastoma
- PMID: 39232046
- PMCID: PMC11375122
- DOI: 10.1038/s41598-024-71462-8
The compartment-specific manipulation of the NAD+/NADH ratio affects the metabolome and the function of glioblastoma
Abstract
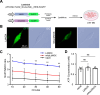
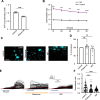
Glioblastoma multiforme (GBM) is the most aggressive type of cancer in the brain and has an inferior prognosis because of the lack of suitable medicine, largely due to its tremendous invasion. GBM has selfish metabolic pathways to promote migration, invasion, and proliferation compared to normal cells. Among various metabolic pathways, NAD (nicotinamide adenine dinucleotide) is essential in generating ATP and is used as a resource for cancer cells. LbNOX (Lactobacillus brevis NADH oxidase) is an enzyme that can directly manipulate the NAD+/NADH ratio. In this study, we found that an increased NAD+/NADH ratio by LbNOX or mitoLbNOX reduced intracellular glutamate and calcium responses and reduced invasion capacity in GBM. However, the invasion was not affected in GBM by rotenone, an ETC (Electron Transport Chain) complex I inhibitor, or nicotinamide riboside, a NAD+ precursor, suggesting that the crucial factor is the NAD+/NADH ratio rather than the absolute quantity of ATP or NAD+ for the invasion of GBM. To develop a more accurate and effective GBM treatment, our findings highlight the importance of developing a new medicine that targets the regulation of the NAD+/NADH ratio, given the current lack of effective treatment options for this brain cancer.
Keywords: LbNOX; Glioblastoma; Glutamate; Invasion; NAD+/NADH.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

