Immunoliposome-based targeted delivery of the CRISPR/Cas9gRNA-IL30 complex inhibits prostate cancer and prolongs survival
- PMID: 39232121
- PMCID: PMC11447253
- DOI: 10.1038/s12276-024-01310-2
Immunoliposome-based targeted delivery of the CRISPR/Cas9gRNA-IL30 complex inhibits prostate cancer and prolongs survival
Abstract
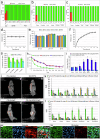
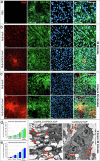
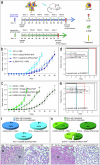
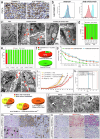
The development of selective and nontoxic immunotherapy targeting prostate cancer (PC) is challenging. Interleukin (IL)30 plays immunoinhibitory and oncogenic roles in PC, and its tumor-specific suppression may have significant clinical implications. CRISPR/Cas9-mediated IL30 gene deletion in PC xenografts using anti-PSCA antibody-driven lipid nanocomplexes (Cas9gRNA-hIL30-PSCA NxPs) revealed significant genome editing efficiency and circulation stability without off-target effects or organ toxicity. Biweekly intravenous administration of Cas9gRNA-hIL30-PSCA NxPs to PC-bearing mice inhibited tumor growth and metastasis and improved survival. Mechanistically, Cas9gRNA-hIL30-PSCA NxPs suppressed ANGPTL 1/2/4, IL1β, CCL2, CXCL1/6, SERPINE1-F1, EFNB2, PLG, PF4, VEGFA, VEGFD, ANG, TGFβ1, EGF and HGF expression in human PC cells while upregulated CDH1, DKK3 and PTEN expression, leading to low proliferation and extensive ischemic necrosis. In the syngeneic PC model, IL30-targeting immunoliposomes downregulated NFKB1 expression and prevented intratumoral influx of CD11b+Gr-1+MDCs, Foxp3+Tregs, and NKp46+RORγt+ILC3, and prolonged host survival by inhibiting tumor progression. This study serves as a proof of principle that immunoliposome-based targeted delivery of Cas9gRNA-IL30 represent a potentially safe and effective strategy for PC treatment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Comment in
-
Immunoliposome targeting IL-30.Nat Rev Urol. 2024 Nov;21(11):649. doi: 10.1038/s41585-024-00958-9. Nat Rev Urol. 2024. PMID: 39390115 No abstract available.
References
-
- Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics. CA Cancer J. Clin.72, 7–33 (2022). - PubMed
MeSH terms
Substances
Grants and funding
- IG 2019 - ID. 23264/Associazione Italiana per la Ricerca sul Cancro (Italian Association for Cancer Research)
- 2014-2020 (PON R&I)/Ministero dell'Istruzione, dell'Università e della Ricerca (Ministry of Education, University and Research)
- ECS00000041/Ministero dell'Istruzione, dell'Università e della Ricerca (Ministry of Education, University and Research)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

