Disordered regions in the IRE1α ER lumenal domain mediate its stress-induced clustering
- PMID: 39232130
- PMCID: PMC11480506
- DOI: 10.1038/s44318-024-00207-0
Disordered regions in the IRE1α ER lumenal domain mediate its stress-induced clustering
Abstract
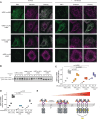
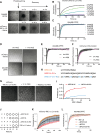
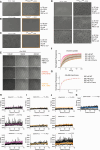
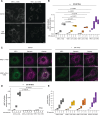
Conserved signaling cascades monitor protein-folding homeostasis to ensure proper cellular function. One of the evolutionary conserved key players is IRE1, which maintains endoplasmic reticulum (ER) homeostasis through the unfolded protein response (UPR). Upon accumulation of misfolded proteins in the ER, IRE1 forms clusters on the ER membrane to initiate UPR signaling. What regulates IRE1 cluster formation is not fully understood. Here, we show that the ER lumenal domain (LD) of human IRE1α forms biomolecular condensates in vitro. IRE1α LD condensates were stabilized both by binding to unfolded polypeptides as well as by tethering to model membranes, suggesting their role in assembling IRE1α into signaling-competent stable clusters. Molecular dynamics simulations indicated that weak multivalent interactions drive IRE1α LD clustering. Mutagenesis experiments identified disordered regions in IRE1α LD to control its clustering in vitro and in cells. Importantly, dysregulated clustering of IRE1α mutants led to defects in IRE1α signaling. Our results revealed that disordered regions in IRE1α LD control its clustering and suggest their role as a common strategy in regulating protein assembly on membranes.
Keywords: Biomolecular Condensates; IRE1; Supported Lipid Bilayers; Unfolded Protein Response.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, Lennon CJ, Kluger Y, Dynlacht BD (2007) XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell 27(1):53–66 - PubMed
-
- Alberti S, Dormann D (2019) Liquid-liquid phase separation in disease. Annu Rev Genet 53:171–194 - PubMed
-
- Alberti S, Hyman AA (2021) Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat Rev Mol Cell Biol 22(3):196–213 - PubMed
MeSH terms
Substances
Grants and funding
- FWF-SFB F79/Austrian Science Fund (FWF)
- FWF-W 1261/Austrian Science Fund (FWF)
- P30231-B,P30415-B,P36572/Austrian Science Fund (FWF)
- SFB grant F79/Austrian Science Fund (FWF)
- 450648163/Deutsche Forschungsgemeinschaft (DFG)
- SFB 1507/Deutsche Forschungsgemeinschaft (DFG)
- N/A/Frankfurt Institute of Advanced Studies
- N/A/The LOEWE Center for Multiscale Modelling in Life Sciences of the state of Hesse
- LM2023042/International Max Planck Research School on Cellular Biophysics
- CZ.02.1.01/0.0/0.0/18_046/0015974/European Regional Development Fund-Project, UP CIISB,
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

