Immune system adaptation during gender-affirming testosterone treatment
- PMID: 39232147
- PMCID: PMC11374716
- DOI: 10.1038/s41586-024-07789-z
Immune system adaptation during gender-affirming testosterone treatment
Erratum in
-
Author Correction: Immune system adaptation during gender-affirming testosterone treatment.Nature. 2024 Oct;634(8033):E5. doi: 10.1038/s41586-024-08081-w. Nature. 2024. PMID: 39317781 Free PMC article. No abstract available.
Abstract
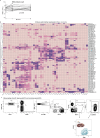
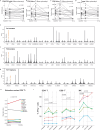
Infectious, inflammatory and autoimmune conditions present differently in males and females. SARS-CoV-2 infection in naive males is associated with increased risk of death, whereas females are at increased risk of long COVID1, similar to observations in other infections2. Females respond more strongly to vaccines, and adverse reactions are more frequent3, like most autoimmune diseases4. Immunological sex differences stem from genetic, hormonal and behavioural factors5 but their relative importance is only partially understood6-8. In individuals assigned female sex at birth and undergoing gender-affirming testosterone therapy (trans men), hormone concentrations change markedly but the immunological consequences are poorly understood. Here we performed longitudinal systems-level analyses in 23 trans men and found that testosterone modulates a cross-regulated axis between type-I interferon and tumour necrosis factor. This is mediated by functional attenuation of type-I interferon responses in both plasmacytoid dendritic cells and monocytes. Conversely, testosterone potentiates monocyte responses leading to increased tumour necrosis factor, interleukin-6 and interleukin-15 production and downstream activation of nuclear factor kappa B-regulated genes and potentiation of interferon-γ responses, primarily in natural killer cells. These findings in trans men are corroborated by sex-divergent responses in public datasets and illustrate the dynamic regulation of human immunity by sex hormones, with implications for the health of individuals undergoing hormone therapy and our understanding of sex-divergent immune responses in cisgender individuals.
© 2024. The Author(s).
Conflict of interest statement
P.B., T.L. and J.M. are cofounders of Cytodelics AB (Stockholm, Sweden), which produces and distributes the whole blood cell stabilizer solutions used in this study. P.B. is an executive board member of Kancera AB, scientific advisor for Pixelgen Technologies AB, Helaina Inc., Scailyte AG, Oxford Immune Algorithmics Ltd, Sention Health AB and the Swedish Olympic Committee. The other authors declare no competing interests.
Figures









References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

