Mechanisms that clear mutations drive field cancerization in mammary tissue
- PMID: 39232148
- PMCID: PMC11374684
- DOI: 10.1038/s41586-024-07882-3
Mechanisms that clear mutations drive field cancerization in mammary tissue
Abstract
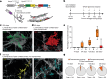
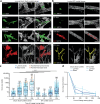
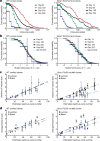
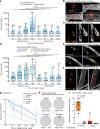
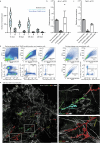
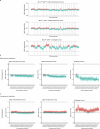
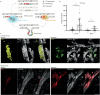
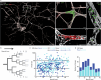
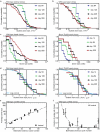
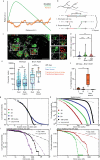
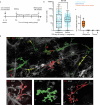
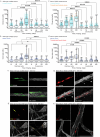
Oncogenic mutations are abundant in the tissues of healthy individuals, but rarely form tumours1-3. Yet, the underlying protection mechanisms are largely unknown. To resolve these mechanisms in mouse mammary tissue, we use lineage tracing to map the fate of wild-type and Brca1-/-;Trp53-/- cells, and find that both follow a similar pattern of loss and spread within ducts. Clonal analysis reveals that ducts consist of small repetitive units of self-renewing cells that give rise to short-lived descendants. This offers a first layer of protection as any descendants, including oncogenic mutant cells, are constantly lost, thereby limiting the spread of mutations to a single stem cell-descendant unit. Local tissue remodelling during consecutive oestrous cycles leads to the cooperative and stochastic loss and replacement of self-renewing cells. This process provides a second layer of protection, leading to the elimination of most mutant clones while enabling the minority that by chance survive to expand beyond the stem cell-descendant unit. This leads to fields of mutant cells spanning large parts of the epithelial network, predisposing it for transformation. Eventually, clone expansion becomes restrained by the geometry of the ducts, providing a third layer of protection. Together, these mechanisms act to eliminate most cells that acquire somatic mutations at the expense of driving the accelerated expansion of a minority of cells, which can colonize large areas, leading to field cancerization.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

