Frontostriatal salience network expansion in individuals in depression
- PMID: 39232159
- PMCID: PMC11410656
- DOI: 10.1038/s41586-024-07805-2
Frontostriatal salience network expansion in individuals in depression
Abstract
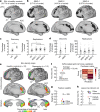
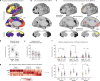
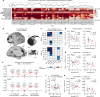
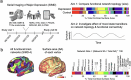
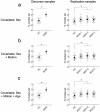
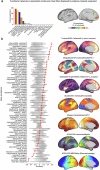
Decades of neuroimaging studies have shown modest differences in brain structure and connectivity in depression, hindering mechanistic insights or the identification of risk factors for disease onset1. Furthermore, whereas depression is episodic, few longitudinal neuroimaging studies exist, limiting understanding of mechanisms that drive mood-state transitions. The emerging field of precision functional mapping has used densely sampled longitudinal neuroimaging data to show behaviourally meaningful differences in brain network topography and connectivity between and in healthy individuals2-4, but this approach has not been applied in depression. Here, using precision functional mapping and several samples of deeply sampled individuals, we found that the frontostriatal salience network is expanded nearly twofold in the cortex of most individuals with depression. This effect was replicable in several samples and caused primarily by network border shifts, with three distinct modes of encroachment occurring in different individuals. Salience network expansion was stable over time, unaffected by mood state and detectable in children before the onset of depression later in adolescence. Longitudinal analyses of individuals scanned up to 62 times over 1.5 years identified connectivity changes in frontostriatal circuits that tracked fluctuations in specific symptoms and predicted future anhedonia symptoms. Together, these findings identify a trait-like brain network topology that may confer risk for depression and mood-state-dependent connectivity changes in frontostriatal circuits that predict the emergence and remission of depressive symptoms over time.
© 2024. The Author(s).
Conflict of interest statement
C.L. and C.J.L. are listed as inventors for Cornell University patent applications on neuroimaging biomarkers for depression which are pending or in preparation. C.L. has served as a scientific advisor or consultant to Compass Pathways PLC, Delix Therapeutics and Brainify.AI. The remaining authors declare no competing interests.
Figures








Update of
-
Expansion of a frontostriatal salience network in individuals with depression.bioRxiv [Preprint]. 2023 Aug 14:2023.08.09.551651. doi: 10.1101/2023.08.09.551651. bioRxiv. 2023. Update in: Nature. 2024 Sep;633(8030):624-633. doi: 10.1038/s41586-024-07805-2. PMID: 37645792 Free PMC article. Updated. Preprint.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

