Global marine microbial diversity and its potential in bioprospecting
- PMID: 39232160
- PMCID: PMC11390488
- DOI: 10.1038/s41586-024-07891-2
Global marine microbial diversity and its potential in bioprospecting
Abstract
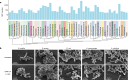
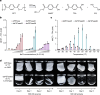
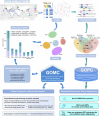
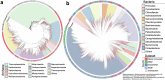
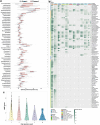
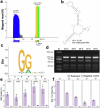
The past two decades has witnessed a remarkable increase in the number of microbial genomes retrieved from marine systems1,2. However, it has remained challenging to translate this marine genomic diversity into biotechnological and biomedical applications3,4. Here we recovered 43,191 bacterial and archaeal genomes from publicly available marine metagenomes, encompassing a wide range of diversity with 138 distinct phyla, redefining the upper limit of marine bacterial genome size and revealing complex trade-offs between the occurrence of CRISPR-Cas systems and antibiotic resistance genes. In silico bioprospecting of these marine genomes led to the discovery of a novel CRISPR-Cas9 system, ten antimicrobial peptides, and three enzymes that degrade polyethylene terephthalate. In vitro experiments confirmed their effectiveness and efficacy. This work provides evidence that global-scale sequencing initiatives advance our understanding of how microbial diversity has evolved in the oceans and is maintained, and demonstrates how such initiatives can be sustainably exploited to advance biotechnology and biomedicine.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures









References
-
- Overmann, J. & Lepleux, C. in The Marine Microbiome (ed. Stal, L. J. & Cretoiu, M. S.) 21–55 (2016).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

