Alternating high-fat diet enhances atherosclerosis by neutrophil reprogramming
- PMID: 39232165
- PMCID: PMC12019644
- DOI: 10.1038/s41586-024-07693-6
Alternating high-fat diet enhances atherosclerosis by neutrophil reprogramming
Abstract
Systemic immune responses caused by chronic hypercholesterolaemia contribute to atherosclerosis initiation, progression and complications1. However, individuals often change their dietary habits over time2, and the effects of an alternating high-fat diet (HFD) on atherosclerosis remain unclear. Here, to address this relevant issue, we developed a protocol using atherosclerosis-prone mice to compare an alternating versus continuous HFD while maintaining similar overall exposure periods. We found that an alternating HFD accelerated atherosclerosis in Ldlr-/- and Apoe-/- mice compared with a continuous HFD. This pro-atherogenic effect of the alternating HFD was also observed in Apoe-/-Rag2-/- mice lacking T, B and natural killer T cells, ruling out the role of the adaptive immune system in the observed phenotype. Discontinuing the HFD in the alternating HFD group downregulated RUNX13, promoting inflammatory signalling in bone marrow myeloid progenitors. After re-exposure to an HFD, these cells produced IL-1β, leading to emergency myelopoiesis and increased neutrophil levels in blood. Neutrophils infiltrated plaques and released neutrophil extracellular traps, exacerbating atherosclerosis. Specific depletion of neutrophils or inhibition of IL-1β pathways abolished emergency myelopoiesis and reversed the pro-atherogenic effects of the alternating HFD. This study highlights the role of IL-1β-dependent neutrophil progenitor reprogramming in accelerated atherosclerosis induced by alternating HFD.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no financial and non-financial competing interests
Figures

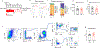


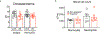




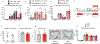





Comment in
-
Lower your cholesterol early, and stick with it!Nat Rev Cardiol. 2025 Feb;22(2):69-70. doi: 10.1038/s41569-024-01095-x. Nat Rev Cardiol. 2025. PMID: 39424909 No abstract available.
References
Additional references
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

